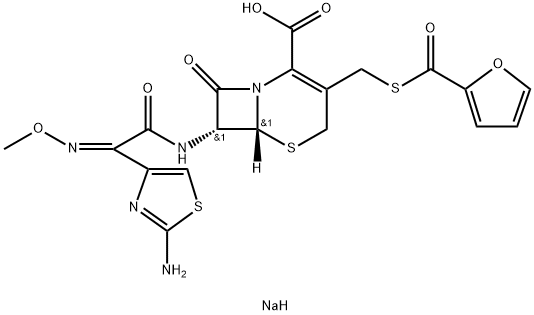
Ceftiofur sodium: Uses, Mechanism of action and Side effects
Uses of Ceftiofur sodium
Ceftiofur sodium is a third-generation cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria, including beta-lactamase-producing strains. It is approved for use in horses only by the intramuscular (IM) route (labelled dose for streptococcal infections is 2.2 to 4.4 mg/kg intramuscularly every 24 hours). In addition, Ceftiofur sodium is also used to treat and control infections caused by susceptible pathogens in cattle and swine. It is registered for the treatment of respiratory disease and interdigital necrosis (foot rot) in lactating cows. Ceftiofur sodium is indicated for the treatment/control of bacterial respiratory disease in pigs (porcine bacterial pneumonia) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Salmonella cholera and Streptococcus suis type 2.

Ceftiofur sodium has been administered on an intramammary basis for treatment of coliform mastitis, but this is an extralabel use. It is used in horses for treatment of streptococcal respiratory infections (registered treatment) and extralabel use for treating other infections such as those caused by gram-negative bacilli, including Escherichia coli, Klebsiella pneumoniae, and salmonella. Higher doses should be used for nonstreptococcal bacteria in horses. Ceftiofur sodium is registered for a daily SQ injection for treatment of UTIs in dogs, but it has not been evaluated for treatment of other infections.
Mechanism of action
Mechanism of antimicrobial activity of Ceftiofur sodium: Ceftiofur sodium binds to and inactivates penicillin-binding protein (PBP), an enzyme involved in the final stages of bacterial cell wall assembly and remodelling of the cell wall during growth and division.Inactivation of PBP interferes with cross-linking of peptidoglycan chains, which are essential for the strength and rigidity of the bacterial cell wall. cross-linking of peptidoglycan chains. This leads to weakening of the bacterial cell wall and cell lysis.
Pharmacokinetics of ceftiofur sodium
The current work was outlined to decide kinetic profile and bioavailability of ceftiofur sodium at measurements of 2.2 mg/kg. b.wt. after a single intravenous and subcutaneous injection in pre-weaned calves. Five clinically typical pre-weaned calves were utilized in this work. Serum ceftiofur concentrations were decided by utilizing high performance liquid chromatography (HPLC) strategy. The serum concentration-time curve has shown a two compartment open model. Taking after a single intravenous injection, distribution half-life (t0.5α) was 0.11 ± 0.01h, volume of distribution (Vdss) was 0.16 ± 0.004 L/kg, elimination half-life (t0.5β) was 11.07 ± 0.12h and total body clearance (CLtot) was 0.013 ± 0.005 L/kg/h. Taking after a single subcutaneous organization, ceftiofur had a peak serum concentration (Cmax) 6.64 ± 0.08 μg/ml at a time (tmax) of 3.38 ± 0.069 h, disposal half-life (t0.5el) was 2.11 ± 0.25h demonstrating the propensity of calves to eliminate ceftiofur in slow rate. Bioavailability was 77.04 ± 1.64% after subcutaneous injection indicating a good absorption of ceftiofur. Ceftiofur serum concentrations along 36 h following subcutaneous injection in the current work were exceeding the MICs of different susceptible microorganisms responsible for serious disease problems. These results demonstrating successful use of ceftiofur in pre-weaned calves.
Side effects of Ceftiofur sodium
There were no adverse systemic reactions to Ceftiofur sodium intramuscular injection, common side effects included mild muscle irritation, diarrhoea, reduced food intake, weight loss, haematological changes associated with acute inflammation and stress, and serum chemistry changes associated with reduced food intake and diarrhoea related to the dose of treatment. Adverse reactions are most severe in the days following initiation of dosing and tend to become less severe at the end of the 10-day dosing period. In addition, some dogs exhibit anaemia and bone marrow changes. In a few cases, allergic reactions have occurred.
References
[1] EL-HEWAITY M. Pharmacokinetics of ceftiofur sodium after intravenous and subcutaneous administration in pre-weaned calves[C]. 2021. DOI:10.21608/EVMSPJ.2021.174688.
[2] GIANLUCA CELANI. Clinical Efficacy of a Single Intravenous Regional Limb Perfusion with Marbofloxacin versus Ceftiofur Sodium to Treat Acute Interdigital Phlegmon in Dairy Cows.[J]. Animals, 2023. DOI:10.3390/ani13101598.
You may like
Lastest Price from Ceftiofur sodium manufacturers
US $0.00-0.00/KG2025-11-29
- CAS:
- 104010-37-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $5.00-0.50/KG2025-05-30
- CAS:
- 104010-37-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS