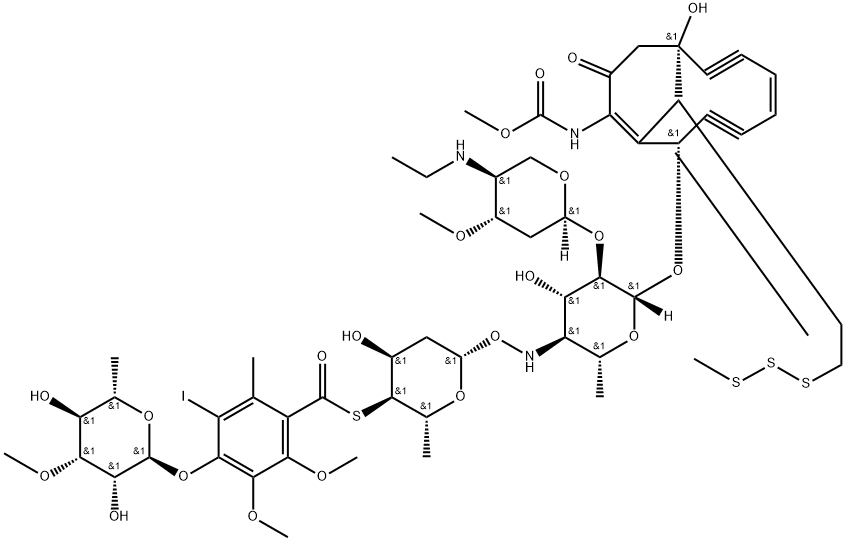
Calicheamicin and Calicheamicin gamma 1I
Introduction
Calicheamicins are bioactive molecules produced by bacteria of the Micromonospora species and constitute a class of powerful enedynes (that is, organic compounds containing two triple bonds and one double bond) endowed with antibiotic effect against both Gram+ and Gram− bacteria and anticancer activity. It is a highly potent antitumor antibiotic initially isolated from the actinomycete Micromonospora echinospora. It binds to the minor groove of DNA and cleaves double-stranded.
Calicheamicin is the active payload in two approved ADCs:
Besponsa, based on the humanized IgG4 Ab Inotuzumab targeting the B cell receptor CD22 and employed for the treatment of relapsed/refractory B cell acute lymphoblastic leukemia, and Mylotarg, based on the humanized IgG4 Ab Gemtuzuma targeting the myeloid cell surface antigen CD33 and used to treat relapsed/refractory acute myeloid leukemia[1].
Mode of action
Once conjugated to an Ab, calicheamicin release relies on a two-step process: first, the hydrazone is selectively released in the acidic intracellular environment, then the disulfide link is reduced by intracellular glutathione; this, in turn, activates an intramolecular 1,4-addition reaction followed by a Bergman cyclization, leading to the formation of radical species. This reactive intermediate binds within the DNA double helix minor groove, ultimately producing DNA double-strand breaks and, hence, inducing cell death.
Calicheamicin gamma 1I
Calicheamicin gamma 1I is a discovered diyne-ene--containing antitumor antibiotic with considerable potency against murine tumors.
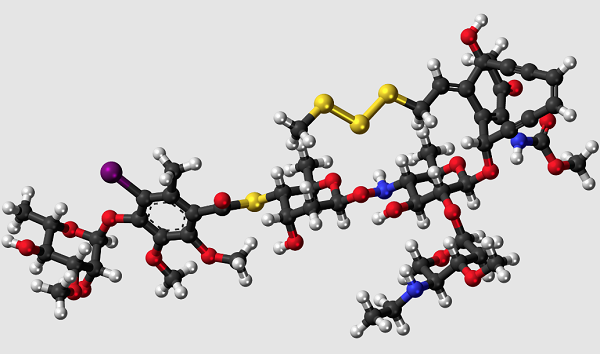
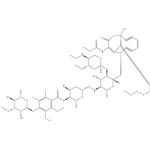
It has been isolated from fermentation media to which sodium iodide has been added. Its chemical structure consists of a core bicyclo (7.3.1) tridecadiynene moiety (ring R) attached to an aryl tetrasaccharide chain. This chain consists of linked elements involving a hydroxylamino sugar (ring A), a thio sugar (ring B), a thiobenzoate (ring C), and a rhamnose sugar (ring D). An ethylamino sugar (ring E) is connected to sugar A through a glycosidic linkage. The enediyne core and attached trisaccharide segments are common to esperamicin A1 (R, A, B, and C rings) and calicheamicin g1 I (R, A, B, and E rings), including the novel NH-O connectivity linking the A and B sugars [2].
In vitro, this drug interacts with double-helical DNA in the minor groove and causes site-specific double-stranded cleavage. It is proposed that the observed cleavage specificity is a result of a unique fit of the drug and DNA followed by the generation of a nondiffusible 1,4-dehydrobenzene--diradical species that initiates oxidative strand scission by hydrogen abstraction on the deoxyribose ring. The ability of calicheamicin gamma 1I to cause double-strand cuts at very low concentrations may account for its potent antitumor activity[3].
References
[1] Cannon, J. “Site-Specific Drug Delivery Utilizing Monoclonal Antibodies.”Advanced and Modern Approaches for Drug Delivery (2023): 649-681.
[2] R A Kumar, D J Patel, N Ikemoto. “Solution structure of the calicheamicin gamma 1I-DNA complex.” Journal of Molecular Biology 265 2 (1997): 187–201.
[3] Nada Zein. “Calicheamicin γ1I: an Antitumor Antibiotic That Cleaves Double-Stranded DNA Site Specifically.” Science 240 4856 (1988).
You may like
Related articles And Qustion
See also
Lastest Price from Calicheamicin manufacturers
US $8.00-1.00/kg2024-04-05
- CAS:
- 108212-75-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available