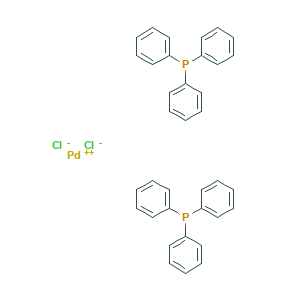Bis(triphenylphosphine)palladium(II) chloride: properties, applications and safety
General Description
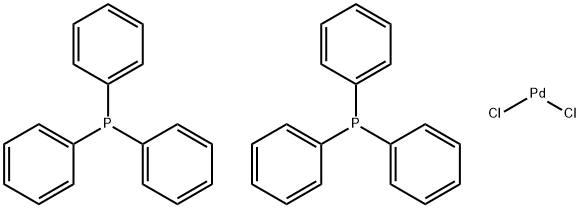
Bis(triphenylphosphine)palladium(II) chloride, with the chemical formula PdCl2(PPh3)2, is a coordination complex consisting of palladium and triphenylphosphine. It serves as a valuable catalyst in organic synthesis, particularly in carbon-carbon cross-coupling reactions like the Suzuki-Miyaura and Heck reactions. This compound also plays a crucial role in the production of antiviral drugs through catalytic coupling reactions. Its application in synthesizing antiviral compounds is essential for the development of effective treatments. In terms of safety, Bis(triphenylphosphine)palladium(II) chloride is considered non-toxic and non-carcinogenic, making it relatively safe for use in organic synthesis. However, caution must be exercised during handling as the compound can absorb moisture and carbon dioxide from the air, resulting in the formation of harmful hydrochloric acid.

Figure 1. Bis(triphenylphosphine)palladium(II) chloride
Properties
Bis(triphenylphosphine)palladium(II) chloride is a coordination complex composed of palladium and triphenylphosphine. It has the chemical formula PdCl2(PPh3)2 and is often used as a catalyst in organic synthesis. This complex is a colorless or white solid that is insoluble in water but soluble in organic solvents. It is stable up to 230°C, and its structure consists of a central palladium atom surrounded by two phosphorus atoms from the triphenylphosphine ligand and two chloride ions. The presence of the chloride ions affects the electronic properties of the complex and is important for its catalytic activity. Bis(triphenylphosphine)palladium(II) chloride is commonly used as a catalyst in carbon-carbon cross-coupling reactions, such as Suzuki-Miyaura reactions and Heck reactions, as well as in hydrogenation reactions and allylic substitution reactions. 1
Applications
Catalyst
Bis(triphenylphosphine)palladium(II) chloride, commonly referred to as Pd(PPh3)2Cl2, is a highly versatile catalyst widely used in various organic synthesis reactions. One of its primary applications is in carbon-carbon cross-coupling reactions, such as the Suzuki-Miyaura and Heck reactions. These reactions play a crucial role in the synthesis of organic molecules, including pharmaceuticals and fine chemicals. The catalytic activity of Pd(PPh3)2Cl2 enables the coupling of two different organic molecules through a carbon-carbon bond formation. This catalyst promotes the reaction by facilitating the oxidative addition of an organic halide or pseudohalide to the palladium center, followed by transmetallation with an organometallic reagent and reductive elimination to form the desired products. In addition to cross-coupling reactions, Pd(PPh3)2Cl2 can also be employed as a catalyst in hydrogenation reactions and allylic substitution reactions. These reactions allow for the selective reduction of multiple bonds or the introduction of substituents at allylic positions, respectively. In summary, Bis(triphenylphosphine)palladium(II) chloride is a versatile catalyst with wide-ranging applications in organic synthesis, particularly in carbon-carbon cross-coupling reactions. Its catalytic activity enables the efficient formation of carbon-carbon bonds and the synthesis of complex organic molecules. 2
Synthesis of antiviral drugs
Bis(triphenylphosphine)palladium(II) chloride plays a crucial role in the synthesis of antiviral drugs through catalytic coupling reactions.The utilization of Bis(triphenylphosphine)palladium(II) chloride in the synthesis of carbocyclic analogs of 5-ethynyl-2-deoxyuridine. This method, reported recently as a means to synthesize the true nucleoside 5-ethynyl-2-deoxyuridine, involves the use of Bis(triphenylphosphine)palladium(II) chloride and copper(I) iodide as catalysts. It facilitates the formation of the key components necessary for the synthesis of antiviral drugs. The carbocyclic analog of the drug has been demonstrated to exhibit inhibitory effects on the replication of type 1 and type 2 herpes simplex viruses in Vero cells. Similarly, the carbocyclic analog of 5-ethynyl-2-deoxyuridine possesses moderate activity against herpes simplex viruses, types 1 and 2. Consequently, the application of Bis(triphenylphosphine)palladium(II) chloride in the synthesis of antiviral drugs is of significant importance as it facilitates crucial reactions involved in the production of effective antiviral compounds. 3
Safety
Bis(triphenylphosphine)palladium(II) chloride is non-toxic and non-carcinogenic, making it a relatively safe compound for use in organic synthesis. However, it is important to handle this compound with caution as it has the potential to absorb moisture and carbon dioxide from the air, leading to the formation of harmful hydrochloric acid. To ensure safety, it is recommended to wear appropriate personal protective equipment such as gloves, masks, eye protection, and lab coats when handling this compound. Furthermore, proper storage conditions should be maintained, storing the compound in a dry and well-ventilated area away from heat and light sources to prevent any unwanted reactions with moisture or light. 4
Reference
1. PubChem. COMPOUND SUMMARY: Bis(triphenylphosphine)palladium (II) chloride. 2005, CID: 84124.
2. Dodero VI, Koll LC, Faraoni MB, Mitchell TN, Podestá JC. Stereoselective synthesis of stannyl enones via palladium-catalyzed and free radical hydrostannation of alkynyl ketones with trineophyltin hydride. J Org Chem, 2003, 68(26):10087-10091.
3. Shealy YF, ODell CA, Arnett G, Shannon WM. Synthesis and antiviral activity of the carbocyclic analogues of 5-ethyl-2-deoxyuridine and of 5-ethynyl-2-deoxyuridine, J Med Chem. 1986, 29(1):79-84.
4. SAFETY DATA SHEET: Bis(triphenylphosphine)palladium(II) chloride. Fisher Scientific, 2009, 1-6.
Related articles And Qustion
See also
Lastest Price from Bis(triphenylphosphine)palladium(II) chloride manufacturers

US $0.00/KG2025-04-15
- CAS:
- 13965-03-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00/KG2025-03-21
- CAS:
- 13965-03-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG /month