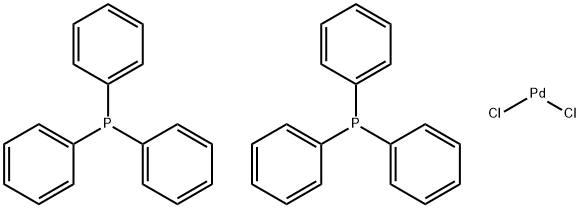
Bis(triphenylphosphine)palladium(II) chloride – a coordination compound of palladium
Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Both cis and trans isomers are known, but the cis isomer is more common. Many analogous complexes are known with different phosphine ligands [1].
Bis(triphenylphosphine)palladium(II) dichloride is an organometallic complex. It is an efficient cross-coupling catalyst for C-C coupling reaction, such as Negishi coupling, Suzuki coupling, Sonogashira coupling and Heck coupling reaction. Detection of bis(triphenylphosphine)palladium(II) dichloride by electrospray ionization quadrupole ion trap mass spectrometry using different imidazolium salts as the charge carrier has been reported. It is employed as catalyst for the Heck reaction medium.
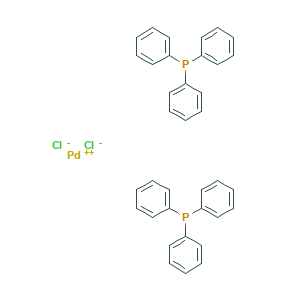
Bis(triphenylphosphine)palladium(II) chloride may be prepared by treating palladium(II) chloride with triphenylphosphine [2]:
PdCl2 + 2PPh3 → PdCl2(PPh3)2
Upon reduction with hydrazine in the presence of excess triphenylphosphine, the complex is a precursor to tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) [3]:
PdCl2(PPh3)2 + 2 PPh3 + 2.5 N2H4 → Pd(PPh3)4 + 0.5 N2 + 2 N2H5+Cl−
Like most Pd(II) complexes, the two different isomers (cis and trans) of the compound have planar structures. However, the cis configuration has slightly distorted planar structure because of the steric bulk of the phosphine ligands.
Bis(triphenylphosphine)palladium(II) dichloride was employed in the following studies:
• As model catalyst for the evaluation of functionalized silica′s for the adsorptive recovery of homogeneous catalysts, via its interaction with metal centre.
• As catalyst in the synthesis of diphenylacetylene.
• One-pot synthesis of furoquinolines, via Pd-catalyzed Sonogashira coupling followed by Cu(I) catalyzed ring closure.
• Catalyst for Sonogashira coupling of aryl alkynes to 2-bromothiazole and 2-bromothiophene [4].
Bis(triphenylphosphine)palladium(II) chloride is used as a pre-catalyst for a variety of coupling reactions [5].
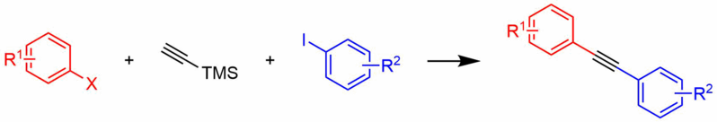
The Suzuki reaction was once limited by high levels of catalyst and the limited availability of boronic acids. Replacements for halides were also found, increasing the number of coupling partners for the halide or pseudohalide as well. Using bis(triphenylphosphine)palladium chloride as the catalyst, triflates and boronic acids have been coupled on an 80 kilogram scale in good yield. The same catalyst is effective for the Sonogashira coupling [1].
References
[1] https://en.wikipedia.org/wiki/Bis(triphenylphosphine)palladium_chloride
[2] Hiroshi Itatani, J.C.Bailar (1967). "Homogeneous Catalysis in the Reactions of olefinic Substances. V.Hydrogenation of Soybean Oil Methyl Ester with Triphenylphosphine and Triphenylarsine Palladium Catalysts". Journal of the American Oil Chemists' Society. 44: 147. doi:10.1007/BF02558176.
[3] D. R. Coulson (1972). Tetrakis(triphenylphosphine)palladium(0). Inorg. Synth. Inorganic Syntheses. 13. pp. 121–124. doi:10.1002/9780470132449.ch23. ISBN 9780470132449.
[4] https://www.sigmaaldrich.com/catalog/product/aldrich/412740?lang=en®ion=US
[5] René Severin, Jessica Reimer, Sven Doye (2010). "One-Pot Procedure for the Synthesis of Unsymmetrical Diarylalkynes". J. Org. Chem. 75 (10): 3518–352. doi:10.1021/jo100460v. PMID 20420397.
You may like
Related articles And Qustion
See also
Lastest Price from Bis(triphenylphosphine)palladium(II) chloride manufacturers

US $0.00/KG2025-04-15
- CAS:
- 13965-03-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00/KG2025-03-21
- CAS:
- 13965-03-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG /month