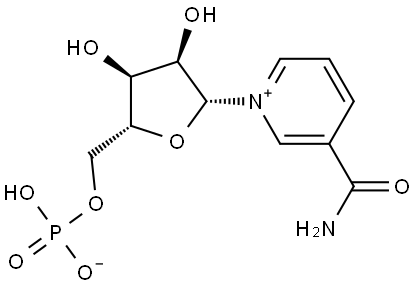
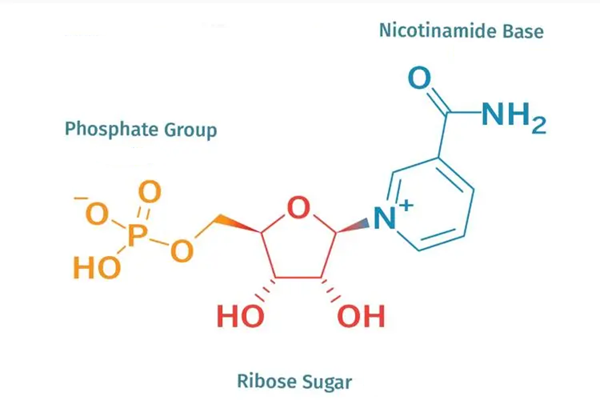
ANH, NPA ask FDA to reverse position on NMN supplements
So far, so longevity, but despite the growing body of scientific evidence supporting the potential benefits of NMN supplementation, the regulatory status of NMN supplements has proven to be a point of contention. In the US, dietary supplements are regulated by the FDA under the Dietary Supplement Health and Education Act (DSHEA) of 1994. Under this law, dietary supplements are generally not subject to pre-market approval by the FDA, as long as they contain ingredients that are “generally recognized as safe” (GRAS) and meet certain other requirements.
However, now the FDA has taken the position that NMN does not meet the definition of a dietary ingredient under DSHEA and should be classified as a drug. This classification would require NMN supplements to undergo more rigorous testing and approval processes – similar to those required for prescription drugs. This is despite NMN being available as supplement for many years.
The Alliance for Natural Health USA (ANH) and Natural Products Association (NPA) asked that FDA make the determination above or “commit to exercise enforcement discretion in connection with the marketing and selling of NMN in or as a dietary supplement.”
You may like
Related articles And Qustion
Lastest Price from β-Nicotinamide Mononucleotide manufacturers

US $130.00-1.00/kg2025-11-03
- CAS:
- 1094-61-7
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 300tons
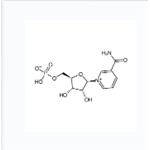
US $1.00/KG2025-10-15
- CAS:
- 1094-61-7
- Min. Order:
- 1000KG
- Purity:
- 99.99%
- Supply Ability:
- 20 TONS