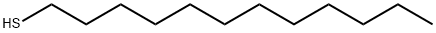1-Dodecanethiol: properties and applications
General Description
1-Dodecanethiol is an organic compound with the chemical formula HS(CH2)11SH and contains a sulfide functional group. It can be used in self-assembled monolayers to protect cobalt surfaces from oxidation. This study demonstrates that 1-Dodecanethiol SAMs can effectively prevent oxidation and corrosion, making them a potential protective coating for cobalt surfaces. The optimization of the self-assembly process can further enhance their performance and stability. Additionally, 1-Dodecanethiol shows promise in extracting noble metal ions from aqueous solutions, providing an alternative method for the extraction of noble metals from various sources. Its application in environmental pollution control and precious metal recovery can greatly contribute to these fields.

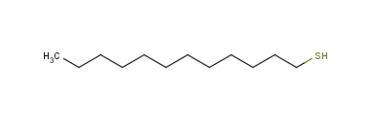
Figure 1. 1-Dodecanethiol
Properties
1-Dodecanethiol is a type of organic compound with the chemical formula HS(CH2)11SH. It is a thiol, meaning it contains a sulfide functional group (-S-). 1-Dodecanethiol has a molecular formula C12H26S and belongs to the class of organic compounds known as long-chain thiols. This compound has several significantproperties. First, it is insoluble in water but soluble in organic solvents such as hexane, toluene, and chloroform. 1-Dodecanethiol is also flammable, with a flash point of 130 degrees Celsius. In terms of its physical state, 1-dodecanethiol is a colorless liquid. It has alow boiling point of approximately 265 degrees Celsius and a density of 0.88 grams per milliliter. Additionally, 1-dodecanethiol exhibits weak antioxidantactivity in vivo. The structure of 1-dodecanethiol features a linear carbon chain with twosulfur atoms attached at the end. This carbon chain is composed of twelve carbons and thus gives the compound its dodecan prefix. The two sulfur atoms that terminate the carbon chain each have a lone pair of electrons, which can participate in hydrogen bonding. The HS(CH2)11SHmolecule has an additional -SH group compared to similar long-chain thiols, which confers on it additionalreactivity. This compound can undergo reactions with other compounds to form covalent bonds, such as thioetherifications with other sulfides or Michael additions with activated olefins. 1
Applications
Self-assembled monolayers
1-Dodecanethiol can be applied in self-assembled monolayers (SAMs) for the protection of cobalt surfaces. Cobalt and its alloys find extensive use across various fields; however, their susceptibility to oxidation poses limitations. Alkanethiol SAMs offer an effective approach to prevent oxidation and corrosion. While this surface modification method has been extensively explored for oxidizable metals like copper and nickel, the use of alkanethiol SAMs on cobalt surfaces has received limited attention. In a research, the self-assembly process of 1-dodecanethiol monolayers on cobalt was studied, focusing on parameters such as surface pretreatment, solvent, immersion time, and concentration. Optimization of each parameter was carried out to achieve a densely packed and stable monolayer capable of effectively inhibiting the reoxidation of cobalt substrates. The resulting monolayers were characterized using X-ray photoelectron spectroscopy (XPS), polarization modulation infrared reflection-absorption spectroscopy, and contact angle measurements. The stability of the optimized 1-dodecanethiol monolayer was confirmed by XPS analysis after exposure to air for 28 days. Overall, this study demonstrates the potential of 1-Dodecanethiol SAMs as a protective coating for cobalt surfaces, providing valuable insights into the optimization of the self-assembly process for enhanced performance and stability. 2
Extraction of noble metal ions
1-Dodecanethiol shows potential in the extraction of noble metal ions from aqueous solutions. A phase-transfer protocol based on 1-Dodecanethiol has been developed for this purpose, facilitating the transfer of noble metal ions from the aqueous phase to the hydrocarbon phase. The protocol involves initially mixing the aqueous solution containing noble metal ions with an ethanol solution of 1-Dodecanethiol, followed by the extraction of the formed coordination compounds between noble metal ions and 1-Dodecanethiol into a non-polar organic solvent. Characterization techniques including inductively coupled plasma atomic emission spectroscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis have demonstrated the effectiveness of this protocol in extracting various noble metal ions from water to dichloromethane, with an extraction efficiency exceeding 96%. Furthermore, the protocol exhibits high selectivity in separating noble metal ions from other transition metal ions. Therefore, this protocol presents an attractive alternative for the extraction of noble metals from water, soil, or waste printed circuit boards. Its application holds great potential in environmental pollution control and precious metal recovery, making it a significant contribution to these fields. 3
Reference
1. PubChem. COMPOUND SUMMARY: 1-Dodecanethiol. National Library of Medicine, 2005, CID:8195.
2. Devillers S, Hennart A, Delhalle J, Mekhalif Z. 1-Dodecanethiol self-assembled monolayers on cobalt. Langmuir, 2011, 27(24):14849-14860.
3. Chen D, Cui P, Cao H, Yang J. A 1-dodecanethiol-based phase transfer protocol for the highly efficient extraction of noble metal ions from aqueous phase. J Environ Sci (China), 2015, 29:146-150.
Related articles And Qustion
Lastest Price from 1-Dodecanethiol manufacturers

US $1.00/KG2025-04-21
- CAS:
- 112-55-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-04-15
- CAS:
- 112-55-0
- Min. Order:
- 20kg
- Purity:
- 98.5%
- Supply Ability:
- 10 tons