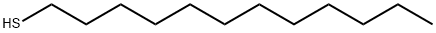1-Dodecanethiol: synthesis, applications in organic synthesis and safety
General Description
1-Dodecanethiol is a sulfur-containing compound with wide industrial applications. Its synthesis can be achieved using a polystyrene-supported sulfonic acid catalyst in water, offering an efficient and green approach for production. In organic synthesis, it is used as a reducing agent, stabilizer for gold nanoparticles, ligand for metal-catalyzed reactions, and building block for surfactants and polymers. It has particular importance in the synthesis of sulfides. However, 1-Dodecanethiol poses several safety risks and requires precautionary measures such as protective clothing and eye protection to minimize exposure.

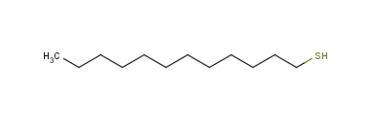
Figure 1. 1-Dodecanethiol
Synthesis
1-Dodecanethiol is an important sulfur-containing compound that has a wide range of industrial applications. Its synthesis involves the conversion of 1-dodecanol to 1-dodecanethiol, which can be achieved using various methods such as reduction with hydrogen sulfide or reaction with thionyl chloride, among others. Recently, a new method for the synthesis of 1-dodecanethiol has been developed using a hydrophobic polystyrene-supported sulfonic acid catalyst. This catalyst has been found to be highly effective for hydrolysis of thioesters in pure water and is much superior to other Bronsted acid catalysts. Furthermore, this catalytic system has been successfully used for transprotection of thiols from thioesters to thioethers in water. The use of water as a solvent makes this method environmentally friendly and economically viable. In summary, the synthesis of 1-dodecanethiol can be achieved using a polystyrene-supported sulfonic acid catalyst in water, offering an efficient and green approach for the production of this important compound. 1
Applications in organic synthesis
1-Dodecanethiol is an organic compound with a thiol functional group that has found numerous applications in organic synthesis. It can be used as a reducing agent for the synthesis of nanoparticles and as a stabilizer for the synthesis of gold nanoparticles. Additionally, it can serve as a ligand for metal-catalyzed reactions and as a building block for the synthesis of surfactants and polymers. One notable application of 1-dodecanethiol is in the synthesis of sulfides. Treatment of alcohols with 1-dodecanethiol in the presence of a strong acid catalyst can lead to the formation of the corresponding thiols, which can then be further reacted to produce sulfides. 1-Dodecanethiol can also be used in combination with other reagents to produce more complex sulfides. For example, the use of trifluoromethanesulfenamide reagent with 1-dodecanethiol can lead to the synthesis of perfluoroalkanesulfenates and perfluoroalkyldisulfides. In conclusion, 1-dodecanethiol is a versatile compound with a range of applications in organic synthesis, particularly in the synthesis of sulfides and as a ligand for metal-catalyzed reactions. Its use in combination with other reagents can lead to the formation of more complex compounds. 2,3
Safety
1-Dodecanethiol is a chemical compound that poses several safety risks. It is harmful if swallowed or inhaled, causing acute toxicity and respiratory difficulties. The compound can also be harmful upon contact with the skin, causing irritation, burns, and allergic reactions. Additionally, it is known to cause serious eye damage and irritation. To ensure safety when handling 1-Dodecanethiol, precautionary measures should be taken. Personal protective clothing and appropriate eye protection should be worn to prevent skin and eye contact. In case of skin contamination, immediate washing is necessary. Wet or contaminated work clothing should be removed and replaced. Symptoms of exposure include nausea, headache, abdominal pain, diarrhea, cough, sore throat, and skin sensitization. Inhalation may result in irritation of the upper respiratory tract. Workers exposed to mixtures containing 1-Dodecanethiol have shown an increase in chromosomal aberrations. Non-human toxicity studies have shown that prolonged contact does not cause skin irritation, but it can lead to epidermal hyperplasia and elongation of hair follicles. Allergic responses have been observed in animals, but sensitization tests gave negative results. In rabbit eyes, undiluted 1-Dodecanethiol caused severe conjunctiva and iris irritation, which persisted even after 7 days. Considering these safety hazards, proper handling procedures and protective measures should be followed to minimize the risks associated with 1-Dodecanethiol. 4
Reference
1. Iimura S, Manabe K, Kobayashi S. Hydrophobic polymer-supported catalyst for organic reactions in water: acid-catalyzed hydrolysis of thioesters and transprotection of thiols. Org Lett. 2003 Jan 23;5(2):101-103.
2. Efficient aerobic oxidation of phosphines, phosphites, and sulfides by using trialkylborane. Bulletin of the Chemical Society of Japan, 2007, 80(11): 2229-2231.
3. Direct Perfluoroalkylthiolation of Few Chalcogenols. Chinese Journal of Chemistry, 2016, 34(5): 455-458.
4. Dodecane-1-thiol. European Chemicals Agency, EC / List no. 203-984-1.
Related articles And Qustion
Lastest Price from 1-Dodecanethiol manufacturers

US $1.00/KG2025-04-21
- CAS:
- 112-55-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-04-15
- CAS:
- 112-55-0
- Min. Order:
- 20kg
- Purity:
- 98.5%
- Supply Ability:
- 10 tons