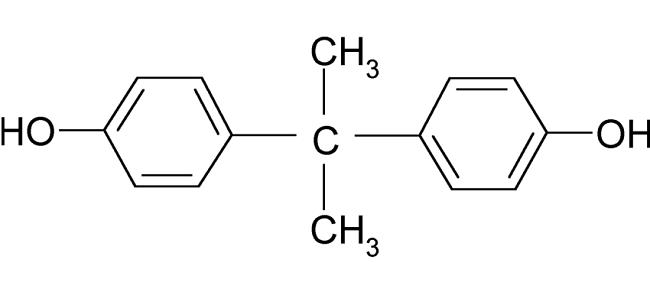
Biological implications of Bisphenol A
Bisphenol A (BPA) is a chemical produced in large quantities for use primarily in the production of polycarbonate plastics and epoxy resins. It is a representative environmental estrogen and also affects a wide array of biological processes, including the metabolic, thyroid hormone, and androgen systems.
Discovery
Bisphenol A was first synthesized in the 1890s. In the early 1930s, Dodds and Lawson discovered that BPA was estrogenic, but less effective than estradiol-17β (E2).Since Dodds et al. synthesized diethylstilbestrol as an effective synthetic estrogen,BPA has never been used as a drug.
Properties
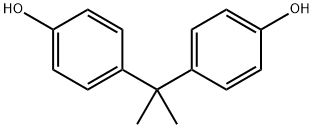
A white solid, molecular formula C15H16O2, Mr. 228.29, density 1.20 g/mL (25°C), melting point 158–159°C. Poorly soluble in water.
Production
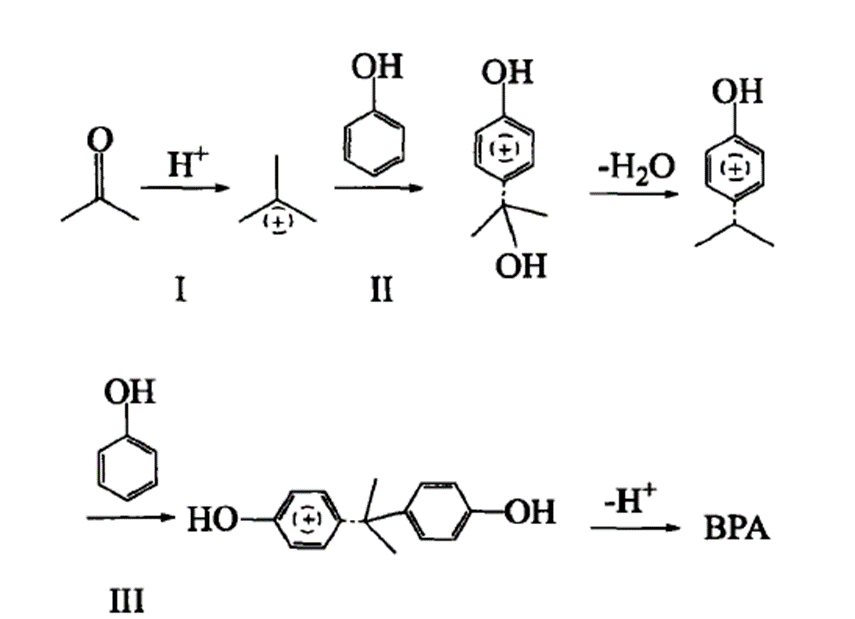
Bisphenol A is synthesized by the condensation of acetone with two equivalents of phenol. Applications BPA is used to make certain polycarbonate plastic and epoxy resins. Polycarbonate plastic is utilized in reusable food and drink containers, including baby milk and water bottles, and in tableware and water pipes. The inner walls of cans and the lids of glass jars and bottles for foods and beverages are lined with epoxy resins as a protective coating. BPA is also found in some polyvinyl chloride plastics and paper products.
Biological and pathophysiological implications
In a mammalian uterotrophic assay, BPA was found to be about 10,000-fold less potent than E2.In the stably transfected human estrogen receptor α (ERα (ESR1)) transactivation in vitro assay, the estrogenic potency of BPA relative to E2 is 0.0034%.In the in vitro reporter gene assay, the relative potency of BPA to E2 for medaka ERα is 0.075%.Thus, BPA is a weak estrogen exhibiting a relatively short half-life, but can build up to concentrations of concern by continuous exposure to it. Developmental animals (i.e., fetuses and young animals) may be more susceptible to BPA (and other endocrine disruptors) exposure than adults. The biological half-life of BPA in humans is estimated to be less than 6 h.
Low-dose effects
Some effects of BPA are observed at extremely low concentrations, and higher concentrations often do not result in the same effects. This dose-response relationship is often referred to as an inverted U response. In 1997, the adverse effects of the low-dose exposure of laboratory animals to BPA were first shown as an increase in the size of the fetal mouse prostate.Some examples of the low dose effects of BPA in rodents include increased postnatal growth, early onset of sexual maturation in females, decrease in daily sperm production and fertility in males, stimulation of mammary gland development in female offspring, altered immune function, decrease in antioxidant enzymes, changes in the brain (including an increase in progesterone receptor mRNA levels), and behavioral effects (including hyperactivity, increase in aggressiveness, and decreased maternal behavior).
You may like
Related articles And Qustion
See also
Lastest Price from Bisphenol A manufacturers

US $6.00/kg2025-04-21
- CAS:
- 80-05-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $999.00-666.00/ton2025-04-21
- CAS:
- 80-05-7
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000