Production and Carcinogenicity of Dichlorodiphenyltrichloroethane
Dichlorodiphenyltrichloroethane (DDT) is a persistent organic pollutant with high bioaccumulation properties. In addition to toxic effects, DDT and its derivatives have estrogenic and/or antiandrogenic properties.

Discovery
DDT was first synthesized in 1873, and its insecticidal action was discovered by M€uller in 1939. M€uller was awarded the Nobel Prize in Physiology or Medicine in 1948 for his discovery of the high efficiency of DDT as a contact poison against several arthropods.
Properties
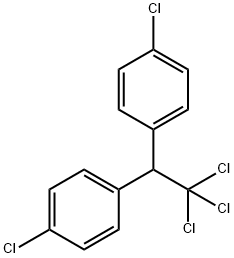
A white, crystalline powder, molecular formula C14H9Cl5, Mr. 354.49. Poorly soluble in water. The term DDT/DDTs is often used to refer to a family of isomers (p,p' -DDT) and their breakdown products (p,p' Ddichlorodiphenyldichloroethylene (DDE), o,p' iDDE, p,p'- Ddichlorodiphenyldichloroethane (DDD).
Production
DDT is produced by the reaction of chloral with chlorobenzene in the presence of sulfuric acid as a catalyst. Applications DDT was used to control insects during World War II, and then as an agricultural insecticide. Almost all uses of DDT were banned in most developed countries in the 1970s–80s. In some countries, DDT was applied to the inside walls of homes to kill or repel mosquitoes. Technical-grade DDT (generally used as an insecticide) contains 65%–80% (active ingredient) of p,p' 8DDT, 15%–21% of o,p'2DDT, and up to 4% of p,p'DDDD.
Biological and pathophysiological implications
Persistent organic pollutants
DDT is one of the POPs (persistent organic pollutants). Once released into the environment, they (1) remain intact for exceptionally long periods of time; (2) become widely distributed throughout the environment as a result of natural processes involving soil, water, and, most notably, air; (3) accumulate in the fatty tissue of living organisms, including humans, and are found at higher concentrations at higher levels in the food chain; and (4) are toxic to both humans and wildlife.
Some POPs, including DDT and its derivatives, are also considered to be endocrine disruptors, which, by altering the hormonal system, can damage the reproductive systems of exposed individuals as well as their offspring. Hormone activities Regarding steroid hormone disruption effects, p,p' eDDT, DDT’s main component, has little or no androgenic or estrogenic activity. o,p' sDDT has estrogenic activity, and the relative potency of o,p' DDDT with respect to estradiol-17β for the medaka estrogen receptor α is 0.16%.4 The DDT metabolite, DDE, can act as an antiandrogen but not as an estrogen.5–8 Of the DDT metabolites, p,p' ODDE is the most potent antiandrogen.
Carcinogenicity
The carcinogenicity of DDT and its metabolites is evident in rodents. DDT was found to increase the incidences of liver and lung tumors, and lymphomas, in mice.