Biocatalytic Conversion of Furfural to Furfurylamine: A Sustainable and Safe Approach from Renewable Sources
General Description
Biocatalytic conversion of furfural to furfurylamine offers a sustainable and efficient alternative to traditional chemical methods, utilizing ω-transaminase enzyme for selective bioamination. Chemoenzymatic synthesis from hemicellulose in biomasses combines chemocatalysis and biocatalysis, demonstrating a green approach for furan-based chemical production. Safety concerns surrounding furfurylamine include its flammability, acute toxicity, and potential health hazards upon exposure. Strict safety protocols, including proper handling, ventilation, and protective measures, are essential. Overall, these studies highlight the potential of biocatalysis and chemoenzymatic strategies for sustainable and safe furfurylamine production from renewable sources.

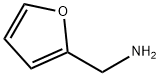
Figure 1. Furfurylamine
Biocatalytic conversion of furfural to furfurylamine
Furfurylamine is a vital component in the production of pharmaceuticals, bioactive chemicals, and fine compounds. Traditionally, Furfurylamine has been obtained through a chemical amination process involving high temperature and pressure, catalyzed by furfural (FAL). However, this method presents drawbacks such as harsh reaction conditions, costly equipment, and potential environmental impact. In contrast, biocatalysis has emerged as a promising alternative due to its mild operating conditions, simplicity, high activity, low corrosion, and eco-friendliness. The enzyme ω-transaminase, dependent on pyridoxamine 5’-phosphate (PLP), exhibits excellent selectivity and can reversibly catalyze the transfer of amino and ketone groups, making it suitable for the bioamination of FAL to produce Furfurylamine. Studies have shown that Escherichia coli expressing ω-transaminase can achieve a 74% yield of Furfurylamine from FAL within 23 hours at 35°C, while cells catalyze the biotransamination of FAL to Furfurylamine with a 100% analytical yield after 3 days at the same temperature. These findings highlight the potential of biocatalytic conversion as a sustainable and efficient approach for Furfurylamine production. 1
Chemoenzymatic catalytic synthesis
Chemoenzymatic catalytic synthesis of furfurylamine from hemicellulose in biomasses represents a sustainable and efficient approach to convert biomacromolecules into valuable furan-based chemicals. This innovative method involves a tandem reaction that combines chemocatalysis and biocatalysis to achieve the desired conversion. Various biomasses such as corncob, bagasse, bamboo shoot shell, corn stalk, rice straw stalk, reed, water bamboo, and sunflower stalk contain different levels of xylan, leading to varying furfural yields after catalysis. By utilizing a shrimp shell-supported solid acid catalyst (Sn-DAT-SS) in a deep eutectic solvent mixture, biomass-derived furfural can be efficiently produced, with corncob yielding the highest furfural content of 52.4%. The catalytic mechanism for converting biomass into furfural using Sn-DAT-SS in the specified solvent was proposed, highlighting the effectiveness of this approach. The study also identified by-products and soluble sugars generated during the process, which can impact the subsequent biotransamination of furfural to furfurylamine. Through the use of E. coli cells expressing ω-transaminase, biomass-derived furfural can be fully aminated to furfurylamine within a timeframe of 24-72 hours. Overall, the chemoenzymatic strategy developed in this study provides a green and eco-friendly system for the conversion of biomasses into valuable furan-based products, demonstrating the potential for sustainable chemical synthesis from renewable sources. 2
Safety
Furfurylamine poses significant health and safety hazards, as outlined by various classification codes and safety guidelines. It is classified as a flammable liquid with the highest degree of flammability, presenting a serious fire hazard. Additionally, its acute toxicity levels are categorized between 2 to 4, indicating potential harm if inhaled, ingested, or upon skin contact. The substance has the potential to cause severe burns to the skin and eyes upon contact, and its combustion can produce irritating, corrosive, and toxic gases. Inhalation of furfurylamine vapors may lead to dizziness or asphyxiation, further emphasizing the acute health risks associated with exposure. In the event of a fire involving furfurylamine, runoff from fire control or dilution water could result in environmental contamination, underscoring the need for careful handling and containment measures. Given these hazards, it is crucial to handle furfurylamine with extreme care, ensuring proper ventilation, personal protective equipment, and adherence to established safety protocols. Strict measures should be in place to prevent exposure through inhalation, ingestion, or skin contact, and appropriate firefighting and spill response procedures must be followed to mitigate risks to both human health and the environment. 3
Reference
1. Feng XQ, Li YY, Ma CL, Xia Y, He YC. Improved conversion of bamboo shoot shells to furfuryl alcohol and furfurylamine by a sequential catalysis with sulfonated graphite and biocatalysts. RSC Adv. 2020;10(66):40365-40372.
2. He W, Ni J, He YC, Ye J. Chemoenzymatic catalytic synthesis of furfurylamine from hemicellulose in biomasses. Int J Biol Macromol. 2022;222(Pt A):1201-1210.
3. Furfurylamine. National Center for Biotechnology Information. 2024. PubChem Compound Summary for CID 3438.
You may like
Related articles And Qustion
See also
Lastest Price from Furfurylamine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 617-89-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $20.00-10.00/kg2025-03-07
- CAS:
- 617-89-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons