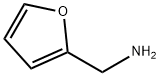
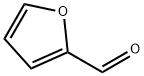
Chemical Properties
Furfurylamine is a colorless to light yellow aromatic amine in liquid form with an ammonia odor. Miscible with water, soluble in ethanol, ether. Deteriorates in air by absorbing carbon dioxide. Derived from furfural based on corn cobs, this green chemistry has proven useful in engine cleaners, and as an intermediate for pharmaceutical, industrial and agricultural chemicals.
Uses
Furfurylamine is used as a water miscible solvent and as an intermediate in manufacturing pharmaceuticals like diuretics, antihypertensive, and antiseptic agents.
Furfurylamine also has use in the synthesis of Barmastine.
2-Furfurylamine is used in the synthesis of 2-Amino-N-(2-furylmethyl)propanamide, as a novel alanylglycine equivalent synthesized by bacilysin synthetase.
Preparation
Synthesis of furfurylamine by Zn/HCl system: To a solution of furfuryloxime (2g, 18mmol) in hydrochloric acid (6.0M, 24ml) was added drop-wise zinc dust (4.71g, 72mmol), and the resultant solution was stirred at room temperature for 2 h. To the resulting slurry was added drop-wise a solution of ammonia (30%, 5.1 mL) and sodium hydroxide (6M, 24mL), the mixture was heated to 60° and stirred for 15mn. After, the resultant solution was cooled and filtered. Then, the mother liquid was extracted with cyclohexane, dried over anhydrous sodium sulfate and filtered. The solvent was removed under vacuum to afford the furfurylamine as a yellow liquid without further purification in 96% of yield (1.68g). The purity determined by NMR was found to be superior to 95%.
SIMPLE, NOVEL SYNTHESIS OF FURFURYLAMINE FROM FURFURAL BY ONE-POT REDUCTIVE AMINATION IN WATER USING ZINC METAL
General Description
Furfurylamine appears as a colorless liquid. About the same density as water. Used as a corrosion inhibitor and to make soldering flux.
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
Amines, such as Furfurylamine, are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Hazard
Flammable, moderate fire risk.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Safety Profile
Poison by
intraperitoneal route. A skin,eye, and
mucous membrane irritant. A dangerous fire
hazard when exposed to heat or flame; can
react with oxidizing materials. To fight fire,
use foam, CO2, drp chemical. When heated
to decomposition it emits toxic fumes of NOx. See also MINES.