Bilirubin: A Key Compound in Biochemical and Clinical Chemistry
What is bilirubin?
Bilirubin is an endogenous compound that is a yellow to orange pigment that may be toxic in some cases, but on the other hand, mild nonconjugated hyperbilirubinemia may prevent cardiovascular disease and tumor development. Under various clinical conditions, serum bilirubin levels typically increase. It is a metabolic product produced by the breakdown of red blood cells in the body. Red blood cells reach the end of their life cycle (usually around 120 days) and are broken down in the spleen and liver, releasing hemoglobin. The heme in hemoglobin is further converted into bilirubin, which is an important component of normal human metabolism[1].

Figure 1 Characteristics of Bilirubin
Bilirubin: chemical structure and formation
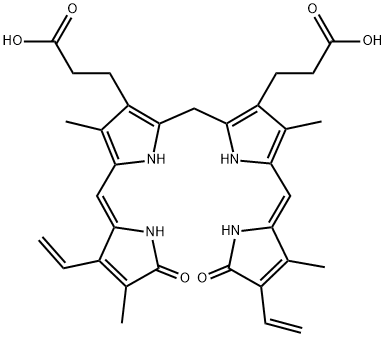
At first glance, bilirubin appears to be a simple molecule. However, the UCB IXα 4Z,15Z molecule, the major compound in mammals, has a peculiar stereo-chemical structure. Indeed, all hydrophilic groups are involved in strong hydrogen bonds, and this turns the molecule into a closed molecule with a ridge-tile conformation. These hydrogen bonds render UCB hydrophobic and they also shield the central –CH2–, which thus becomes inaccessible for the diazo-reagent. Depending on the pH of the plasma, bile, or urine, UCB can be present as an uncharged diacid, as a monoanion, or as a dianion. The uncharged diacid is by far the dominant species at low and physiological pH (>80%) but the ionized fractions become more important in an alkaline milieu because the pK'a values have been determined to be 8.12 and 8.44, respectively, for the first and for the second anion[2].
Bilirubin is formed from haem by the opening of the haem ring at the α carbon bridge. This cleavage is catalyzed by the enzyme haem-oxygenase, and results in the liberation of iron, and in the formation of carbon monoxide and biliverdin IXα. The latter is reduced by a cytosolic enzyme biliverdin-reductase to bilirubin IXα. The haem-oxygenase can temporarily be inhibited by mesoporphyrins, and this suppression results in a decreased UCB production as was shown in neonates. Cleavage at non-α sites is possible; it is probably non-enzymic and occurs only to a minor extent. This results in the formation of other isomers; some can be detected in body fluids, although always in small amounts or under special conditions. The IXβ isomer is present in neonatal urine and in meconium, whereas the IXβ and IXγ isomers have been detected in Gunn rat bile. Because intramolecular hydrogen bonds cannot be formed in these isomers, they are more hydrophilic and appear in urine or bile as an unconjugated pigment. Till now, IXδ has not been demonstrated in mammals. Phototherapy, used in the treatment of neonatal jaundice or in Crigler–Najjar disease, leads to the formation of another group of more hydrophilic derivatives of the natural UCB IXα, such as the 4E,15Z, and the 4Z,15E, and 4E,15E photoisomers, which can be excreted in bile without conjugation.
Bilirubin metabolism under normal conditions
Bilirubin derives from haem present in hemoglobin and is released during the breakdown of senescent erythrocytes, whereas approximately 20% of the daily production is derived from haem proteins such as the cytochrome P 450 isoenzymes, myoglobin, etc. It is formed in the monocytic macrophages of the spleen and bone marrow and in hepatic Kupffer cells and is released in plasma. Per 24 h 3.8 mg/kg or approximately 250–300 mg bilirubin is formed in a normal adult. More is formed in the neonate.
Entry into the hepatocyte appears to be partly passive and partly mediated by organic anion transporter proteins (OATP 1B1 has the highest binding affinity). The role played by OATPs has not yet been clarified quantitatively. In the hepatocytic cytosol, UCB is mostly bound to glutathione-S-transferase A (ligandin), and a small part is bound to the fatty acid-binding protein. As in serum, this binding keeps the free fraction (which is potentially toxic) low.
Bilirubin is conjugated in hepatocytic microsomes in an ester linkage with sugar moieties donated by uridine diphosphate (UDP) sugars. The discovery of glucuronide conjugation of bilirubin was one of the milestones toward understanding bilirubin metabolism and was made almost simultaneously by three groups. The conjugation is catalyzed by UDP-glucuronyltransferase (UDP-GT), an enzyme encoded by the UGT1A1 gene. Both ligandin and UDP-GT appear to be tightly regulated by the nuclear constitutive androstane receptor (CAR). In humans, conjugation occurs mainly with glucuronic acid, but glucose and xylose conjugates are also present in normal bile. The latter are more abundant in cats, dogs, and rodents. One or two sugar moieties are coupled to the –COOH of the propionic acid side chain(s) of UCB in an ester linkage, resulting in monoconjugated or diconjugated bilirubin respectively. The esterification disrupts the intramolecular hydrogen bonds, thereby opening the molecule and rendering the conjugated bilirubin (CB) more water-soluble or amphipathic, allowing excretion in the bile. Conjugation also decreases the binding to albumin or to intracellular proteins 5–10-fold and prevents intestinal re-absorption, because hydrophilic agents do not easily pass the intestinal wall. In addition, the central –CH2– now becomes available for direct attack by the diazo-reagent.
The bilirubin conjugates formed in the hepatocytes are excreted in bile against a concentration gradient and mediated by the canalicular membrane transporter multidrug resistance-related protein 2 (MRP2) also termed ABC-C2, belonging to the adenosine triphosphate (ATP)-binding cassette family. The conjugates are incorporated into mixed micelles (with bile acids, phospholipids, and cholesterol) and pass with the bile into the intestine, where reductive breakdown into urobilinogens occurs by intestinal or bacterial enzymes. A minor part undergoes deconjugation mainly by bacterial enzymes, and the ensuing UCB can undergo intestinal re-absorption, in contrast to CB[2].
![Article illustration]() Reference
Reference
[1] McDonagh A F. Is bilirubin good for you?[J]. Clinics in perinatology, 1990, 17(2): 359-369.
[2] Fevery J. Bilirubin in clinical practice: a review[J]. Liver International, 2008, 28(5): 592-605.
Related articles And Qustion
See also
Lastest Price from Bilirubin manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 635-65-4
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 10 KGS

US $1.00/KG2025-04-21
- CAS:
- 635-65-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt