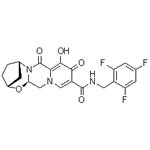
Bictegravir: a novel integrase strand transfer inhibitor
Description
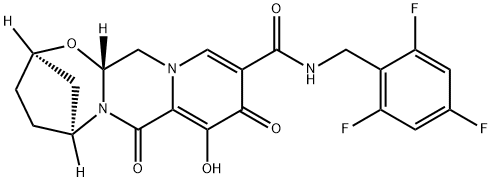
Bictegravir is a novel integrase strand transfer inhibitor(INSTI) as a treatment for HIV-1 infection. HIV integrase is vital in viral replication, catalyzing two consecutive reactions—3' processing and strand transfer—that facilitate the insertion of the reverse-transcribed viral genome into the host cell genome[1].
This drug, discovered by Gilead, was approved in 2018 as part of a combination therapy involving bictegravir, emtricitabine, and tenofovir alafenamide for the treatment of HIV-1 infections. This was based on a phase 3 clinical trial where combining bictegravir, emtricitabine, and tenofovir alafenamide was better tolerated than previous single-tablet regimens.
Synthetic method
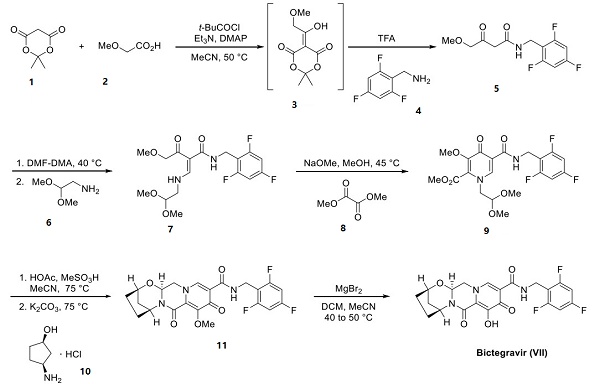
Process chemists at Gilead have disclosed a seven-step route to bictegravir. The approach allowed for the late-state installation of chiral aminocyclopentanol 10. The synthesis began with condensation of Meldrum's acid (1) and methoxyacetic acid 2 in the presence of pivaloyl chloride, giving rise to intermediate 3. The subjection of 3 to benzylamine 4 and TFA ultimately furnished β-ketoamide 5. Treatment with DMF−DMA followed by condensation with dimethyl acetal 6 furnished vinylogous amide 7, making way for a cyclization reaction to generate pyridone 9 by treatment with dimethyl oxalate 8 and sodium methoxide[2]. Acetal deprotection was followed by treatment with syn-aminopentanol 78 under basic conditions. This annulation arose from amide bond formation between primary amine 10 and the methyl ester within 9, followed by condensation with the pendant acetal, allowing for arrival at aminal 11, establishing the polycyclic core of bictegravir. Magnesium bromide mediated demethylation furnished bictegravir.
Pharmacodynamic
Bictegravir was highly active against laboratory and clinical isolates of HIV-1 in vitro [50% effective concentration (EC50) of 0.02–6.6 nmol/L; protein-adjusted 95% effective concentration against laboratory strains of 361 nmol/L], displaying activity against HIV-1 isolates of all groups (M, N and O) and subtypes A–G (EC50 < 0.05–1.71 nmol/L). Notably, bictegravir, like the earlier second-generation INSTI dolutegravir, was significantly (p < 0.05) more active than the first-generation INSTIs elvitegravir and raltegravir against recombinant HIV-1 strains derived from INSTI-naïve patients[3].
Bictegravir also displayed in vitro activity against HIV-1 strains with mutations conferring resistance to elvitegravir and raltegravir, with EC50 values of < 5 nmol/L for all viruses with single INSTI resistance-associated mutations (RAMs) [e.g. Y143R, Q148R] and < 5 or < 10 nmol/L for the majority of those with double or triple INSTI RAMs (e.g. E92Q/N155H ± G163R).
References
[1] Markham, Anthony. “Bictegravir: First Global Approval.” Drugs 78 5 (2018): 601–606.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
[3] Deeks, Emma D. “Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection.” Drugs (2018): 1817–1828.
You may like
Related articles And Qustion
See also
Lastest Price from Bictegravir manufacturers

US $0.00-0.00/g2025-04-21
- CAS:
- 1611493-60-7
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 10kgs
US $0.00-0.00/g2025-04-18
- CAS:
- 1611493-60-7
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 4kg