The application of bictegravir, emtricitabine, and tenofovir alafenamide.
Biktarvy
Biktarvy is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV infection in adults and children weighing at least 31 lb (14 kg) and who meet specific requirements, as determined by a health care provider. It is a complete HIV treatment regimen and should not be used with other HIV medicines. This drug contains three medicines: bictegravir, emtricitabine, and tenofovir alafenamide[1].
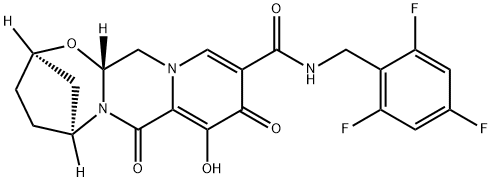
Bictegravir is called an integrase strand transfer inhibitor-INSTI. Emtricitabine is called a nucleoside reverse transcriptase inhibitor, while tenofovir alafenamide is called a reverse transcriptase inhibitor. Emtricitabine and tenofovir alafenamide are often called NRTIs.
Bictegravir
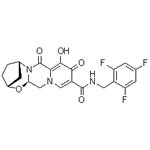
Bictegravir is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2R,5S,13aR)-8-hydroxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid with the amino group of 2,4,6-trifluoro benzylamine. It is a second-generation integrase strand transfer inhibitor (INSTI), the fourth in this class of agents that target the viral integrase and is used (as its sodium salt) for treating HIV-1. It has a role as an HIV-1 integrase inhibitor. It is a conjugate acid of a bictegravir(1-). Bictegravir is used only in combination with other antiretroviral agents in the treatment of HIV infection, and it has had limited use. Bictegravir is associated with a low rate of serum aminotransferase elevations during therapy but has not been linked to instances of acute, clinically apparent liver injury.
Emtricitabine
Emtricitabine (EmtrivaTM) has been recently approved by the US FDA for use in combination with other antiretroviral agents for the treatment of HIV infection. The chemical structure is 5-Fluoro-1-[2R,5S)-2-(hydroxymethyl)-[1,3]oxathiolane-5-yl]cytosine (FTC). It has a similar chemical structure to lamivudine. Following zidovudine (AZT), didanosine(ddI), zalcitabine (ddC), stavudine (d4T), lamivudine (3TC) and abacavir (ABC), emtricitabine (also known as (一)FTC) is the seventh nucleoside reverse transcriptase inhibitor (NRTI) to be approved for the treatment of HIV. Unlike AZT,ddI, ddC, d4T, and ABC, but like 3TC, emtricitabine is an L-nucleoside analog. Although structurally and mechanistically similar to 3TC, emtricitabine appears to be more potent in terms of anti-HIV activity. This is also reflected by more efficient inhibition of HIV-1 reverse transcriptase (RT) by (一)FTC 5'-triphosphate as compared to 3TC 5'-triphosphate. In addition, the RTresistance mutation (M184V) that emerges following the use of 3TC is also seen with (一)FTC but, seemingly, at a lower frequency[2].
Tenofovir alafenamide
Tenofovir alafenamide (TAF) is a prodrug of tenofovir. The antiviral properties of tenofovir are due to its inhibition of HBV polymerase, which, in turn, inhibits DNA synthesis and viral replication. TAF is indicated for the treatment of chronic hepatitis B in adults with compensated liver disease. The Health Canada–recommended dose for TAF is one 25 mg tablet once daily, taken with or without food.
The combination of bictegravir, emtricitabine, and tenofovir AF is used to treat human immunodeficiency virus (HIV) infection in certain adults and children weighing at least 55 pounds (25 kg) who have not received antiretroviral treatment in the past or who have been stable on other antiretroviral treatment(s). Bictegravir is in a class of medications called integrase strand transfer inhibitors (INSTIs). Emtricitabine and tenofovir AF are in a class of medications called nucleoside reverse transcriptase inhibitors (NRTIs). The combination of bictegravir, emtricitabine, and tenofovir AF works by decreasing the amount of HIV in the body. Although bictegravir, emtricitabine, and tenofovir AF will not cure HIV, these medications may decrease your chance of developing acquired immunodeficiency syndrome (AIDS) and HIV-related illnesses such as serious infections or cancer. Taking these medications along with practicing safer sex and making other lifestyle changes may decrease the risk of getting or transmitting the HIV to other people.
References
[1] Harris, Marianne. “What did we learn from the bictegravir switch studies?” Lancet Hiv 5 7 (2018): e336–e337.
[2] Clercq, E. “Emtricitabine.” Drugs 63 1 (2012): 2425–2426.
Related articles And Qustion
See also
Lastest Price from Bictegravir manufacturers

US $0.00-0.00/g2025-04-21
- CAS:
- 1611493-60-7
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 10kgs
US $0.00-0.00/g2025-04-18
- CAS:
- 1611493-60-7
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 4kg