The Synthetic method and Drug interaction of Sarecycline
Description
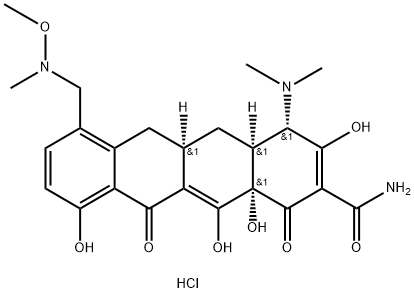
Sarecycline is a novel tetracycline-derived drug specifically designed for acne, with a narrow antibacterial spectrum compared to previously available broad-spectrum tetracycline-class antibiotics. The USFDA approved it for the oral treatment of inflammatory lesions of non-nodular, moderate-to-severe acne vulgaris in patients at least 9 years old. Sarecycline was discovered at Paratek Pharmaceuticals and licensed to Warner Chilcott, which Allergan later acquired. Allergan completed the development and launch of the drug before rights were acquired by Almarall S.A. in 2018[1].
Synthetic method
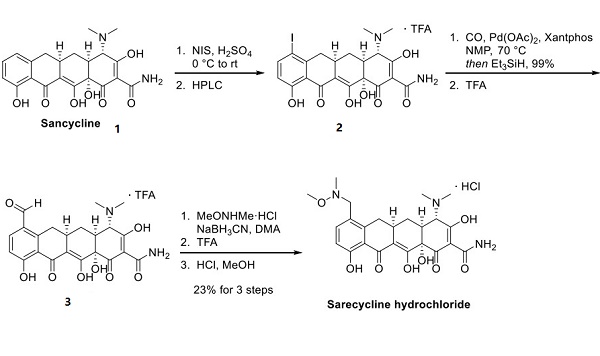
Sarecycline Hydrochloride is the hydrochloride form of sarecycline. The only reported synthetic route to the drug, performed on a small scale by Paratek, is described below. Iodination of commercially available sancycline 1 with N-iodosuccinimide followed by HPLC purification provided iodosancycline 2 as the trifluoroacetate salt[2]. Carbonylation of iodosancycline in the presence of palladium acetate and Xantphos, followed by treatment with triethyl silane, provided the corresponding aldehyde, which was treated with trifluoracetic acid to provide formylsancycline trifluoroacetate 3. Condensation of formylsancycline trifluoroacetate with N, O-dimethylhydroxylamine hydrochloride, reduction with sodium cyanoborohydride, and treatment with hydrochloric acid provided sarecycline hydrochloride in 23% yield for the three-step sequence.
Drug interaction
Sarecycline is a ribosomal protein inhibitor of the tetracycline class that displays potent activity against P. acnes and other Gram-positive bacteria in vitro. The drug has also demonstrated anti-inflammatory effects in vitro. These properties are consistent with those of other tetracyclines, although the exact mechanism by which sarecycline acts to treat acne vulgaris is currently unknown. Sarecycline (like other tetracyclines) may impact the bactericidal effects of penicillin; coadministration should therefore be avoided. Coadministering sarecycline with oral retinoids should also be avoided, as tetracyclines and oral retinoids can increase intracranial pressure. Plasma prothrombin activity may be reduced by sarecycline, which could elevate the bleeding risk of patients taking anticoagulants; the dosage of the anticoagulant may therefore need to be reduced[3].
Pharmacokinetics
Sarecycline reaches maximum plasma concentrations (Cmax) in a median time of 1.5–2.0 h; the drug reaches a steady state by day 7 and has a mean accumulation ratio of 1.5- to 1.6-fold with repeat dosing. Sarecycline can be taken with or without food, although administration of the drug with a milk-containing meal high in fat and calories reduced exposure to the drug by ≈ 30% and delayed the time to Cmax by ≈ 0.53 h, consistent with some other tetracycline-class drugs.
References
[1] Angela Yen Moore. “Sarecycline: A Review of Preclinical and Clinical Evidence.” Clinical, Cosmetic and Investigational Dermatology (2020): 553–560.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
[3] Deeks, Emma D. “Sarecycline: First Global Approval.” Drugs 79 3 (2019): 325–329.
See also
Lastest Price from Sarecycline Hydrochloride manufacturers

US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/kg2025-06-08
- CAS:
- 1035979-44-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg