BIBW2992 Dimaleate - Pharmacodynamics
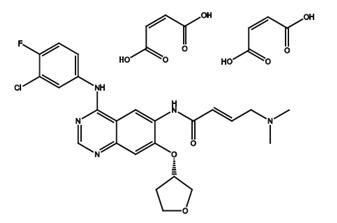
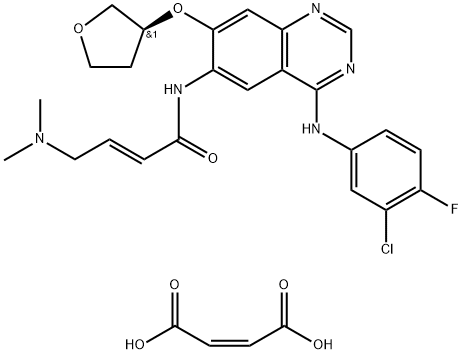
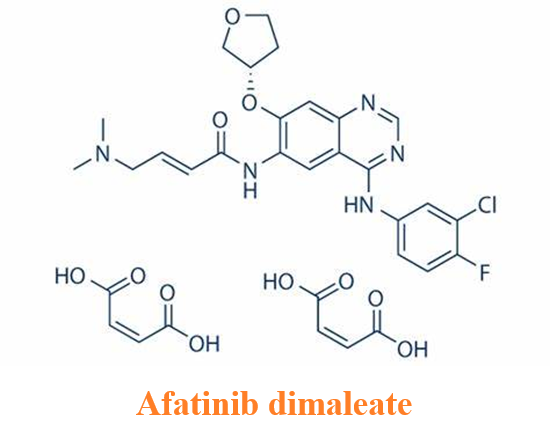
Afatinib dimaleate (Tovok; BIBW2992; Gilotrif) is a salt form of Afatinib. Afatinib is a second-generation, orally administered, irreversible inhibitor of the ErbB family of tyrosine kinases.
Afatinib downregulates ErbB signalling by covalently binding to the kinase domains of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER) 2 and HER4, resulting in irreversible inhibition of tyrosine kinase autophosphorylation; it also inhibits transphosphorylation of HER3. Afatinib is approved as monotherapy for the treatment of EGFR tyrosine kinase inhibitor (TKI)-naïve adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) with activating EGFR mutations in the EU, and for the first-line treatment of patients with metastatic NSCLC whose tumours have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations as detected by a US FDA-approved test in the US[1].
In two randomized, open-label, multinational phase III trials, progression-free survival was significantly prolonged with Afatinib compared with pemetrexed plus cisplatin (LUX-Lung 3) or gemcitabine plus cisplatin (LUX-Lung 6) in treatment naïve patients with advanced NSCLC with activating EGFR mutations[2]. The objective response rate was significantly higher with Afatinib than with pemetrexed plus cisplatin or gemcitabine plus cisplatin, and patient-reported outcomes for symptoms such as cough and dyspnoea and certain healthrelated quality of lifemeasures significantly favoured Afatinib versus pemetrexed plus cisplatin or gemcitabine plus cisplatin. Afatinib also showed efficacy in EGFR TKI- naïve patients with advanced lung adenocarcinoma and activating EGFR mutations who had received no more than one prior chemotherapy regimen for advanced disease, according to the results of the noncomparative, multinational, phase II LUX-Lung 2 trial[3]. Oral Afatinib had a manageable tolerability profile. EGFR-mediated adverse events (e.g. diarrhoea, rash/acne) were generally managed using dose reduction and delays.
In conclusion, Afatinib is a valuable new option for use in treatment- naïve or EGFR TKI- naïve patients with advanced lung adenocarcinoma and activating EGFR mutations[4-5].
References
1.Liao B-C, Lin C-C, Yang JC-H. First-line management of EGFRmutated advanced lung adenocarcinoma: recent developments[J]. Drugs. 2013;73(4):357–69.
2.Nelson V, Ziehr J, Agulnik M, et al. Afatinib: emerging nextgeneration tyrosine kinase inhibitor for NSCLC[J]. Onco Targets Ther. 2013;6:135–43.
3.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models[J]. Oncogene. 2008;27(34):4702–11.
4.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of Afatinib (BIBW 2992), an irreversible ErbB family blocker[J]. J Pharmacol Exp Ther. 2012;343(2):342–50.
5.Chen G, Kronenberger P, Teugels E, et al. Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: the effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab[J]. BMC Med. 2012;10:28.
You may like
Related articles And Qustion
Lastest Price from Afatinib dimaleate manufacturers

US $0.00/g2025-04-21
- CAS:
- 850140-73-7
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g
US $0.00-0.00/g2025-04-20
- CAS:
- 850140-73-7
- Min. Order:
- 10g
- Purity:
- 99% HPLC
- Supply Ability:
- 10000