Benzyl Acrylate: Interchain Transfer Constant Determination
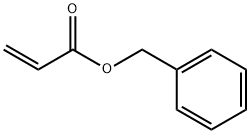
Benzyl acrylate, also known as benzyl ester of acrylic acid, is an important organic synthetic monomer with CAS number 2495-35-4, molecular formula C₁₀H₁₀O₂, and molecular weight 162.19. As a member of the acrylate family, its structural characteristics combining a benzene ring with an ester group confer key roles across multiple industrial sectors. Physically and chemically, benzyl acrylate typically appears as a colourless to pale yellow transparent liquid with a density of approximately 1.06 g/cm³, a boiling point of 110–111°C (8 mmHg), a flash point exceeding 108°C, and a refractive index of 1.5180. It readily dissolves in organic solvents such as alcohols and ethers. Although nominally miscible with water, industrial applications predominantly utilise organic phase systems. In terms of preparation, industrial synthesis primarily involves the esterification reaction between acrylic acid and benzyl alcohol, or employs an ester exchange process using benzyl acetone and acrylic esters. The reaction must be conducted under acidic catalyst conditions to enhance yield. Following distillation purification, the product achieves a purity exceeding 97% (as determined by GC analysis), meeting the requirements for diverse applications. Applications of Benzyl acrylate span multiple industrial contexts: within the coatings and inks industry, it serves as a copolymerising monomer to enhance resin adhesion and weather resistance; in adhesive production, it boosts bonding strength and flexibility; and within the photopolymerisation materials sector, it constitutes a core component of UV coatings. Furthermore, it functions as a pharmaceutical intermediate for synthesising bioactive molecules such as glutamate carboxypeptidase inhibitors.

Direct Determination of Interchain Transfer Constants of Benzyl Acrylate to Poly(Ethyl Acrylate)
Chain transfer reactions are important side reactions in the radical polymerization of acrylates, especially at high temperature, and have great influence on the structures and properties of acrylate polymers. It is generally believed that intrachain transfer is dominant in chain transfer reactions and the reaction rate constant is considerably higher than that of interchain transfer. Different from the entropy separation mechanism of SEC, polymer chromatography based on enthalpy interactions can achieve separation of polymers with different chemical compositions. These chain transfer constants not only reflected the rate of interchain transfer of acrylic monomers, but also are important kinetic parameters in copolymerization systems of acrylic monomers. However, the influence of operating conditions were not perfect, such that more experimental verification and sensitivity analysis are needed. The aim of this paper was to study the effects of conversion, solvent content, polymer and RAFT agent dosages on the determination of the interchain transfer constant of benzyl acrylate to poly(ethyl acrylate).[1]
Using RAFT end groups as an internal standard, the relative mole content of interchain transfer products (termed as the rate of interchain transfer) was quantitatively determined, such that the interchain transfer constants of monomer to another polymer were directly estimated without other kinetic parameters. UV detection in GEPC was facilitated by selecting Benzyl acrylate as the second monomer, for its benzyl ring. Considering the compatibility of the polymerization system and separation effects in GEPC, poly(ethyl acrylate) (PEA) was selected as the chain transfer matrix. Although BzA can easily dissolve PEA, the compatibility of PBzA and PEA was not good when the PBzA MW increased. Thus, the uniformity of the polymerization solution of PEA and BzA became poor with increased Benzyl acrylate conversion, which could hinder contact between the two kinds of polymer. To solve this problem, butyl acetate with low chain transfer activity was added as a solvent to ensure uniformity of the whole reaction system. Experimental results also showed that short chain radical effects were obvious in interchain transfer, but the ratios of monomer to polymer and solvent amounts had little influence on these results, which indicated that this method possessed good stability. Using this method, the interchain transfer constants (Ctr) of benzyl acrylate to poly(ethyl acrylate) were determined at different temperatures, with values in the order of 1–2 × 10−4 and the activation energy of the interchain transfer constant Ctr at 47.8 kJ mol−1.
References
[1] Xiaohua Li. (2021). Direct Determination of Interchain Transfer Constants of Benzyl Acrylate to Poly(Ethyl Acrylate) by RAFT Polymerization and Polymer Chromatography. Macromolecular Chemistry and Physics, 222 12.
See also
Lastest Price from Benzyl acrylate manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 2495-35-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00/kg2025-04-15
- CAS:
- 2495-35-4
- Min. Order:
- 20kg
- Purity:
- 99.5%
- Supply Ability:
- 10 toms