5-Phenyltetrazole: Alkylation Reactions and Key Properties
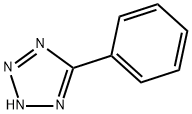
5-Phenyltetrazole is a significant member of nitrogen-containing heterocyclic compounds, CAS Number 18039-42-4, molecular formula C₇H₆N₄, molecular weight 146.149. Its structure features a benzene ring directly connected to a tetrazole five-membered ring. This unique configuration endows it with dual characteristics of both stability and reactivity. Two primary preparation methods exist: firstly, through the reaction of benzonitrile with hydrazine (or hydrazide salts), followed by treatment with nitrous acid; The second is the industrially prevalent ring-closing addition method, employing benzonitrile and sodium azide as reactants. Under zinc catalysis at 100°C for four hours, the product undergoes acid washing, extraction, and column chromatography purification. Applications of 5-phenyltetrazole span three core domains: in pharmaceuticals as a key intermediate for next-generation cephalosporin antibiotics and angiotensin-converting enzyme inhibitors; in photosensitive materials as a stable enhancing the storage stability of silver halide photo-sensitive substances; and in metal protection as a rust inhibitor, corrosion retardant, and lubricant deactivator, effectively reducing metal oxidation losses.

Ortho-Hydroxybenzylation and Alkoxymethylation of 5-Phenyltetrazole
In a search for convenient alkylating methods in the tetrazole chemistry we focused our attention on the application of Mannich bases as the alkylating agents. It is well known that nucleophilic nitrogen-containing molecules may be used to replace the Mannich base amino moieties if those latter are good leaving groups. Succinimide is generally recognized as a good leaving group o and claimed to act as an amine reactant in the Mannich reactions with 2-aza-3-oxoindolizidine3) and benzotriazole). It seemed therefore interesting to investigate whether succinimide reacts with formaldehyde and the typical H- active substrates and, if so, whether the resulting Mannich bases can alkylate the tetrazole ring. Our first experiments were performed with phenols which are well known Mannich substrates; moreover, some phenolic Mannich bases, namely N-(alkoxybenzyl)piperidines, I have been earlier used for the benzylation of 5-phenyltetrazole derivatives.[1]
The reaction of 5-phenyltetrazole and succinimide(equimolar amounts) with paraformaldehyde (a 5 0 % excess) in a 2-chlorophenol solution gave a 52% yield (isolatedpure product) of the expected 2-(2-hydroxy-3-chloroben-zyl)-5-phenyltetrazole. reaction was regioselective, whereas mixtures of 1,5- and2,5-disubstituted tetrazoles (50-77% total yields) werereported to form with N- (alkoxybenzyl)piperidines as the alkylating agents. With no succinimide added, the same reactants even on prolonged heating at 60-70°C gave no TLC-detectable trace of any reaction, thus confirming the role of succinimide. Although alcohols have been claimed once as the H-ac-tive substrates in the synthesis of urea-form aldehyde resins and related products, they usually do not undergo O-aminomethylation in the reaction with aminesand aldehydes and are therefore used as solvents of choice in the Mannich reactions. The 0-alkylation of alcohols has been achieved only with a few Mannich-base methiodides or oxides in the reaction with the appropriate sodium alkoides.
Methylation of 5-Phenyltetrazole
From the reaction of equivalent quantities of methyl iodide and 5-phenyltetrazole in alkaline solution Elpern and Nachodl isolated in 56%yield a product which they considered to be 2-methyl-5- phenyltetrazole since its melting point (41.9-46.9) differed from that of the previously known 1- methyl-5-phenyltetrazole2 (m.p.103-104).Al- though the melting point range for their product was very broad, they did not report any attempts either to improve its purity, or to detect the 1- methyl isomer which might have been formed simultaneously in the methylation. A somewhat similar situation exists in some work by Mihina and Herbst, who studied the reaction of potassium 5-phenyltetrazole with p-nitrobenzyl bromide and benzyl bromide. Although these latter authors stated that the structures of their products were not unequivocally established, their results seemed to indicate preferential alkylation at the 2-position on the ring. We have found, however, that the methylation of 5-phenyitetrazole in alkaline solution consistently yields two isomeric compounds which can be separated by careful fractional crystallization. One of these obtained in about 2000 yield, is identical with 1-methy1-5-phenyltetrazole; the other isomer is formed in about 80 yield and, when free of the 1- methyl derivative, melts sharply at 50.5-51. These results indicate that methylation does occur predominantly, but not exclusively, on the 2-position.
2-Methyl-5-phenyltetrazole and 1-Methyl-5-phenyltetrazole. A solution of 8.25g of methyl iodide (0.058 mol) in 95 ml of acetone was added to a cold solution of 8.3 g of 5- phenyltetrazole (0.057 mol) and 4.65g of sodium hydroxide (0.116 mol) in 23 ml of water. The mixture was refluxed for two hours; at the end of one hour an additional 8.25 g. of methyl iodide was added to make up for losses due to evaporation. The solution was cooled, mixed with 100 ml of benzene, and washed with water until the washings were no longer alkaline. After the benzene layer had been dried over calcium chloride, the solvent was evaporated to yield 9.1 g.(quantitative)of soft, yellow crystalline material; m.p.41-46°. The mixture of isomers was dissolved in 75 ml of diethyl ether and 10 ml of benzene filtered, and the filtrate treated with 25 ml of petroleum ether. Overnight cooling at 0 yielded 1.34 g of colorless needles,m.p.103-104. A mixed melting point with an authentic sample of 1-methyl-5-phenyltetrazole was 103-104.Addition of more petroleum ether to a permanent turbidity and chilling gave 0.45 g more of this same isomer,m.p.101-103. The total yield amounted to 19.7%of theory. The 2-methy1-5-phenyltetrazole was recovered by evaporating the mother liquors to about 40 ml decolorizing with Norite A, fltering, and chilling the filtrate. Long, coarse needles and prisms crystallized slowly; the first crop weighed 2.55 g and melted at 50.Additional,less pure, material could be obtained by evaporating the filtrate to dryness; the total recovery was about 80%of theory. One recrystallization from Skellysolve B raised the melting point to 51°.
References
[1] A. F. H. Hachiam, J. P. (1994). ORTHO-HYDROXYBENZYLATION AND ALKOXYMETHYLATION OF 5-PHENYLTETRAZOLE. Synthetic Communications, 24 1, 665–670.
[2] Henry, R. A. (1951). Methylation of 5-Phenyltetrazole. Journal of the American Chemical Society, 73 9, 4470.
See also
Lastest Price from 5-Phenyltetrazole manufacturers

US $6.00/kg2025-04-21
- CAS:
- 18039-42-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $6.00/kg2025-03-28
- CAS:
- 18039-42-4
- Min. Order:
- 1kg
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/Month