Benzoyl peroxide: Medical use, mechanism and side effects
General description
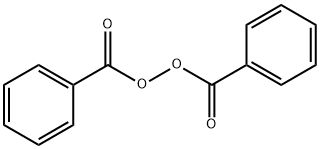
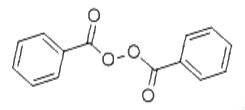
Benzoyl peroxide is often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (C6H5−C(=O)−, Bz) groups connected by a peroxide (−O−O−). Benzoyl peroxide is an oxidizer, which is principally used as in the production of polymers. As a bleach, it has been used as a medication and a water disinfectant.[1] As a medication, benzoyl peroxide is mostly used to treat acne, either alone or in combination with other treatments. Some versions are sold mixed with antibiotics such as clindamycin. It is on the WHO List of Essential Medicines, and, in the US, it is available as an over-the-counter and generic medication. It is also used in dentistry for teeth whitening. Benzoyl peroxide is also used for bleaching flour, hair, and textiles It is also used in the plastics industry. Its appearance is as follows:

Figure 1 The appearance of Benzoyl peroxide
Medical use
Benzoyl peroxide is effective for treating acne lesions. It does not induce antibiotic resistance.[2] It may be combined with salicylic acid, sulfur, erythromycin or clindamycin (antibiotics), or adapalene (a synthetic retinoid). Two common combination drugs include benzoyl peroxide/clindamycin and adapalene/benzoyl peroxide, an unusual formulation considering most retinoids are deactivated by peroxides. Combination products such as benzoyl peroxide/clindamycin and benzoyl peroxide/salicylic acid appear to be slightly more effective than benzoyl peroxide alone for the treatment of acne lesions. Benzoyl peroxide for acne treatment is typically applied to the affected areas in gel, cream, or liquid, in concentrations of 2.5% increasing through 5.0%, and up to 10%. No strong evidence supports the idea that higher concentrations of benzoyl peroxide are more effective than lower concentrations.[2]
Mechanism
Classically, benzoyl peroxide is thought to have a three-fold activity in treating acne. It is sebostatic, comedolytic, and inhibits growth of Cutibacterium acnes, the main bacterium associated with acne.[2-3] In general, acne vulgaris is a hormone-mediated inflammation of sebaceous glands and hair follicles. Hormone changes cause an increase in keratin and sebum production, leading to blocked drainage. C. acnes has many lytic enzymes that break down the proteins and lipids in the sebum, leading to an inflammatory response. The free-radical reaction of benzoyl peroxide can break down the keratin, therefore unblocking the drainage of sebum (comedolytic). It can cause nonspecific peroxidation of C. acnes, making it bactericidal, and it was thought to decrease sebum production, but disagreement exists within the literature on this.[3] Some evidence suggests that benzoyl peroxide has an anti-inflammatory effect as well. In micromolar concentrations it prevents neutrophils from releasing reactive oxygen species, part of the inflammatory response in acne.
Side effects
Application of benzoyl peroxide to the skin may result in redness, burning, and irritation. This side effect is dose-dependent. Because of these possible side effects, it is recommended to start with a low concentration and build up as appropriate, as the skin gradually develops tolerance to the medication. Skin sensitivity typically resolves after a few weeks of continuous use.[4] Irritation can also be reduced by avoiding harsh facial cleansers and wearing sunscreen prior to sun exposure. One in 500 people experience hypersensitivity to BPO and are liable to suffer burning, itching, crusting, and possibly swelling. About one-third of people experience phototoxicity under exposure to ultraviolet (UVB) light.
Benzoyl peroxide breaks down in contact with skin, producing benzoic acid and oxygen, neither of which is very toxic. The carcinogenic potential of benzoyl peroxide has been investigated. A 1981 study published in the journal Science found that although benzoyl peroxide is not a carcinogen, it does promote cell growth when applied to an initiated tumor. The study concluded, "caution should be recommended in the use of this and other free radical-generating compounds".[5] A 1999 IARC review of carcinogenicity studies found no convincing evidence linking BPO acne medication to skin cancers in humans. However, some animal studied found that the compound could act as a carcinogen and enhance the effect of known carcinogens.
References
[1]Stellman JM (1998). Encyclopaedia of Occupational Health and Safety: Guides, indexes, directory. International Labour Organization. p. 104.
[2]Simonart T (December 2012). Newer approaches to the treatment of acne vulgaris. American Journal of Clinical Dermatology. 13 (6): 357–64.
[3]Cotterill JA (1980-01-01). "Benzoyl peroxide". Acta Dermato-Venereologica. Supplementum. Suppl 89: 57–63.
[4]Worret, W.-I.; Fluhr, J. W. (April 2006). Topische therapie mit benzoylperoxid, antibiotika und azelains?ure [Acne therapy with topical benzoyl peroxide, antibiotics and azelaic acid]. Journal der Deutschen Dermatologischen Gesellschaft (in German). 4 (4): 293–300.
[5]Slaga TJ, Klein-Szanto AJ, Triplett LL, Yotti LP, Trosko KE (August 1981). Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 213 (4511): 1023–5.
You may like
Related articles And Qustion
See also
Lastest Price from Benzoyl peroxide manufacturers

US $0.00-0.00/kg2025-09-03
- CAS:
- 94-36-0
- Min. Order:
- 20kg
- Purity:
- 38-41%; 49%MIN; 48-52%; 49-51%; 72-76%; 75%
- Supply Ability:
- 20 tons

US $1000.00-3000.00/kg2025-04-17
- CAS:
- 94-36-0
- Min. Order:
- 1000kg
- Purity:
- 0.75
- Supply Ability:
- 100 Metric Ton/Metric Tons per Year