Arbidol: History, Pharmacokinetics and Toxicity
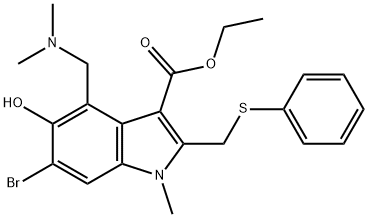
Arbidol (ARB) is a Russian‐made indole‐derivative small molecule licensed in Russia and China for the prevention and treatment of influenza and other respiratory viral infections. ARB also has inhibitory effects on many other viruses such as severe acute respiratory syndrome coronavirus, Coxsackie virus, respiratory syncytial virus, Hantaan virus, herpes simplex virus, and hepatitis B and C viruses.

Pharmacokinetics
Arbidol is absorbed quickly and spreads to organs and tissues. The maximum blood level (concentration) after taking a 50 mg dose occurs 1.2 hours after administration; for a 100 mg dose, 1.5 hours after administration. Arbidol is metabolized in the liver. The half-life of the drug in the body is 17-21 hours. About 40% is excreted in unchanged form, mostly through bile (38.9%) and an insignificant amount through the kidneys (0.12%). Within the first 24 hours after administration, 90% of the drug has left the body.
Uses
Arbidol is used for prevention and treatment in adults and children: --Influenza A and B, Avian or Bird Flu, RSV, SARS (including exacerbation of bronchitis and pneumonia)--Secondary immunodeficiency--Overall therapy for chronic bronchitis, pneumonia and recurrent herpes infections --Prevention of post-operative infections and stabilization of immune status--Overall therapy of acute rotavirus- type intestinal infections in children over 2 years old.
Toxicity
Accorading to related study, the no-observed-adverse-effect-level for 4-week oral administration to rats was considered 200 mg/kg/day, based on clinical observations, pathological findings, body-weight losses, and liver-weight changes.
References
[1] Julie Blaising , Eve-Isabelle Pécheur, Stephen J. Polyak .“Arbidol as a broad-spectrum antiviral: An update.”Antiviral research 107 (2014): Pages 84-94.
You may like
Related articles And Qustion
Lastest Price from Arbidol manufacturers

US $0.00-0.00/kg2024-11-26
- CAS:
- 131707-25-0
- Min. Order:
- 1kg
- Purity:
- 99%HPLC
- Supply Ability:
- 20 tons

US $80.00/KG2023-08-16
- CAS:
- 131707-25-0
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 20tons