Arbidol: mechanism of action, clinical applications and safety
General Description
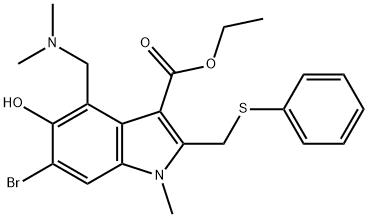
Arbidol is a broad-spectrum antiviral drug that inhibits different stages of the virus life cycle, making it a potential treatment option for various viral infections. Its mechanisms of action include interfering with virus entry and membrane fusion, as well as downregulating N-glycans on the cell surface. Arbidol has primarily been studied for its efficacy against respiratory viral infections such as influenza A and B viruses, and has shown some activity against other respiratory viruses. It is generally well tolerated with common side effects being mild and transient. Arbidol is a safe and well-tolerated drug, with no serious drug-related adverse events reported in human trials.
Figure 1. Capsules of arbidol
Mechanism of action
Arbidol is a broad-spectrum antiviral drug that inhibits different stages of the virus life cycle, such as cell entry, attachment, internalization, and replication. It plays a direct role in removing the influenza virus and can also be used as a host-targeting agent. Arbidol interferes with virus entry or the membrane fusion of virus and host to block virus invasion. During the early stages of infection, after attaching to HA receptors on the cell membrane, the influenza virus is internalized into clathrin-coated vesicles and taken into the host cell via clathrin-mediated endocytosis. Arbidol impairs clathrin-mediated endocytosis by preventing the release of vesicles or interrupting dynamin-2-induced membrane rupture, leading to the accumulation of clathrin-coated vesicles or failure of viral transportation in cells, inhibiting virus infection at the entry step. Recently, a novel antiviral mechanism of Arbidol was revealed where it downregulates SA-linked N-glycans on the cell surface by obstructing the expressions of sialyltransferases, thus inhibiting virus rolling, gliding, and entry. Mechanistically, Arbidol interferes with membrane fusion and increases the pH of the intracellular environment, making the virus unable to complete the process of membrane fusion and release the virus genome, resulting in effective inhibition of virus infection. 1
Clinical applications
Arbidol is an antiviral medication that has shown promising clinical applications. It was first developed in Russia and has been approved for use in several countries, including China. Arbidol has primarily been studied for its efficacy against respiratory viral infections, particularly influenza A and B viruses. Clinical trials have demonstrated the effectiveness of Arbidol in reducing the severity and duration of symptoms associated with influenza infections. It has also shown some activity against other respiratory viruses, such as respiratory syncytial virus (RSV) and rhinovirus. In addition to its antiviral properties, Arbidol is believed to have immunomodulatory effects. It has been reported to enhance the immune response against viral infections, possibly through the activation of certain immune cells. Arbidol has been widely prescribed in China as a preventive measure during influenza outbreaks and as a treatment for mild to moderate cases. It is generally well-tolerated, with common side effects being mild and transient. However, it is important to note that the clinical evidence supporting the widespread use of Arbidol is still limited, and further research is needed to fully evaluate its efficacy and safety in different patient populations. 2
Safety
Arbidol has demonstrated a high safety profile in animal toxicity studies. Biochemical and pathological abnormalities were not observed in rats, guinea pigs, rabbits, and dogs after oral administration of Arbidol for 2-6 months. The oral administration of Arbidol in rats did not affect reproductive function or fetal growth and development. In human clinical trials, Arbidol has been well tolerated with no serious drug-related adverse events reported over the years of its use in Russia and China. Adverse reactions were mainly gastrointestinal symptoms, followed by dizziness, which improved after discontinuation of the drug. Other clinical trials in the treatment of SARS-CoV-2 infection have also shown no severe adverse events during treatment, with some patients experiencing mild gastrointestinal symptoms. No patients discontinued treatment prematurely, indicating that Arbidol is a safe and well-tolerated drug. Overall, Arbidol has a favorable safety profile and can be considered a valuable option for the treatment of viral infections. 3
Reference
1. Blaising J, Polyak SJ, Pécheur E‐I. Arbidol as a broad‐spectrum antiviral: an update. Antiviral Res. 2014;107:84‐94.
2. Kang Y, Shi Y, Xu S. Arbidol: The current demand, strategies, and antiviral mechanisms. Immun Inflamm Dis. 2023 Aug;11(8):e984.
3. Amani B, Amani B, Zareei S, Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID-19: A systematic review and meta-analysis. Immun Inflamm Dis. 2021 Dec;9(4):1197-1208.
Related articles And Qustion
See also
Lastest Price from Arbidol manufacturers

US $0.00-0.00/kg2024-11-26
- CAS:
- 131707-25-0
- Min. Order:
- 1kg
- Purity:
- 99%HPLC
- Supply Ability:
- 20 tons

US $80.00/KG2023-08-16
- CAS:
- 131707-25-0
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 20tons