Applications of 3-Fluoro-4-(4-methylpiperazin-1-yl)aniline
3-Fluoro-4-(4-methylpiperazin-1-yl)aniline is an important organic building block to synthetize substituted aniline products.
Applications
The following example is about its application on the synthesis of pyrrolopyrimidine kinase inhibitors [1]
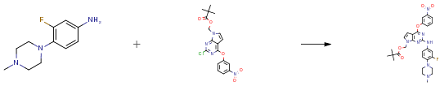
A 500-L, glass-lined reactor was put under an inert atmosphere with nitrogen gas, then was charged with t-butanol (147 kg) under vacuum. Agitation was initiated, and the reactor was heated to 40 ± 5 and nitrogen gas was again introduced. To the reactor was added (2-chloro-4- (3-nitrophenoxy) -7H-pyrrolo [2, 3-d] pyrimidin-7-yl) methyl pivalate (19.10 kg) , anhydrous K2CO3 (32.02 kg) , tris (dibenzylideneacetone) dipalladium (Pd2 (dba) 3 0.88 kg) , dicyclohexyl (2', 4', 6'-triisopropylbiphenyl-2-yl) phosphine (XPhos 0.90 kg) , and 3-fluoro-4- (4-methylpiperazin-1-yl) aniline (9.70 kg) . The resulting mixture was heated at 85 ± 5 for 6 h. The mixture was cooled to 60 ± 5 and then filtered through a pad of diatomaceous earth (4.9 kg) at 50. The reactor was washed with ethyl acetate (36 kg). The diatomaceous earth pad was slurried with ethyl acetate for 30 min at 50 (two times) and then was filtered. The above procedure was repeated and the filtrates were all combined to afford 351 kg of ethyl acetate solution (assay amount 50.36 kg of title compound).
The ethyl acetate solution was concentrated to dryness. Ethyl acetate (281 kg) was added and the mixture was stirred for 30 min to dissolve the residue. Silica gel (37 kg) was then added and the resulting mixture was stirred for 1 h. The mixture was filtered and the resulting filtrate was charged into a 1000-L glass-lined reactor. The solution was washed with purified water (125 kg ×2) and then brine (125 kg) . The organic layer was concentrated to dryness at 45 ± 5 at a vacuum pressure below 0.02 MPa. Ethanol (124 kg) was added to the residue and the resulting mixture was heated at 85 ± 5 for 1 h. Heptane (70 kg) was added and the resulting mixture was heated at 85 ± 5 and stirred for 1 h. The mixture was cooled to 5 ±5 and then was stirred for 5 h. The resulting precipitate was centrifuged. The solid was washed with heptane (20 kg) and dried at 50 ± 5 at a pressure below 0.02 MPa for 16 h to give the product (46.46 kg, 86.4, 99.21 HPLC purity) as an orange solid.
The following example is about its application on the synthesis of nitrogenous heterocyclic derivatives [2]

To a solution of 3-fluoro-4-(4-methylpiperazin-l -yl)aniline (11.01 g, 52.60 mol) in anhydrous CH2C12(200 mL) was added trimethylaluminum (158 mL, 158 mmol, 1.0 M in toluene) slowly under N2. The mixture was stirred at rt for 20 min, followed by the addition of a solution of methyl 3-acetaminocrotonate (9.92 g, 63.10 mmol) in anhydrous CH2C12(20 mL). The reaction mixture was stirred at rt for another 5 h, then quenched with saturated NH4CI aqueous solution and extracted with CH2C12. The combined organic phases were washed with brine, dried over anhydrous Na2S04and concentrated in vacuo. The residue was purified by a silica gel column chromatography (CH2C12/ MeOH (V / V) = 5 : 1) to give the title compound as a white solid (11.31 g, 68%).
The following example is about its application on the synthesis of nitrogenous heterocyclic derivatives [3]

To a solution of 3-fluoro-4-(4-methylpiperazin-l-yl)aniline (0.63 g, 3.01 mmol) in DCM (15 mL) was added trimethylaluminium (5.3 mL, 10.6 mmol, 2 M in toluene) and the mixture was stirred at rt for 0.5 h. A solution of (Z)-methyl-3-(2-(3-fluorophenoxy)acetamido)but-2-enoate (0.80 g, 2.99 mmol) in DCM (5 mL) was added slowly and the resulting mixture was stirred at rt for 12 h. The mixture was then quenched with saturated NH4C1 aqueous solution and extracted with CH2C12 (50 mL × 2). The combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by a silica gel column chromatography (PE / EtOAc (V / V) = 1: 1) to give the product as a pale yellow solid (0.94 g, 73 percent).
References
1.Acea Biosciences, Inc; Acea Pharmaceutical Research Co., Ltd. Mao L, Liu J, Chen Y, Hua Y, Dai K, Xu W, Wang X. Pharmaceutical salts, physical forms, and compositions of pyrrolopyrimidine kinase inhibitors, and methods of making same. WO2017/59702[P], 2017, A1, Paragraph 00220-00221
2.Sunshine Lake Pharma Co., Ltd. Zhang Y, Zhang J, Wang X, Lin R, Cao S, Wang Z, Li J. Nitrogenous heterocyclic derivatives and their application in drugs. WO2014/12360[P], 2014, A1, Paragraph 00294.
3.Sunshine Lake Pharma Co., Ltd. Zhang Y, Zhang J, Wang X, Lin R, Cao S, Wang Z, Li J. Nitrogenous heterocyclic derivatives and their application in drugs. WO2014/12360[P], 2014, A1, Paragraph 00497.
![97511-04-1 3-bromodibenzo[b,d]thiopheneapplication on the synthesis](httpss://www.chemicalbook.com/NewsImg/2019-12-26/20191226164936135017.png)
