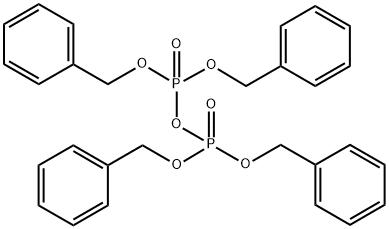
Application research of tetrabenzyl pyrophosphate
Introduction
Tetrabenzyl pyrophosphate (TBPP;Figure 1) has been used frequently in the synthesis of numerous phosphate-containing biologically active molecules and prodrugs. This inexpensive reagent is primarily used for O- or N-phosphorylation with organolithium and other strong bases, although titanium alkoxides have also proven to be selective and effective as Lewis acid catalysts. Recently, Huters et al. utilized tetrabenzyl pyrophosphate in the development of a scalable synthesis of foslevodopa. By employing the previously unreported use of DBU as a base, the group avoided problems associated with stronger bases, enabling isolation of the corresponding dibenzyl phosphate in excellent yield.[1]

Debenzylation for Phosphate Synthesis
The synthetic utility of tetrabenzyl pyrophosphate for achieving chemoselective phosphorylation of phenols, as well as primary, secondary, and tertiary alcohols, is reported. Luke et al. conducted a comprehensive investigation of the capabilities of phosphorylation utilizing tetrabenzyl pyrophosphate, as well as the chemoselective nature and substrate tolerance of Et3SiH-mediated deprotection. This assessment, exemplified by the phosphorylation and debenzylation of more than 30 compounds, highlights both the significant utility and reasonable limitations of these methodologies. Additionally, they introduce a rapid, mild, and chemoselective debenzylation procedure, enabling access to phosphates in the presence of redox sensitive groups. Finally, stoichiometrically controlled monodebenzylation provides a versatile platform for late-stage divergent synthesis of phosphodiester and phosphoramidate chemical libraries.[1]
A new class of neutral carrier responsive to lead ion
Tetrabenzyl pyrophosphate showed the best response to Pb2+, being almost free from interference by other metal cations. By adding the ionic additive potassium tetrakis(p-chlorophenyl)borate (KTpClPB; 40mol% relative to tetrabenzyl pyrophosphate), a drastic change occurred in the electrode response,showing a monovalent species probably due to the formation of PbA+, where A stands for anions present in the sample solution, and decreased the selectivity of the electrode to other metal cations.[2]
Tetrabenzyl pyrophosphate and diphenylphosphinic anhydride, with two phosphoryl groups (P=O) as ligating sites, can be used as novel ionophores to make Pb2+ -selective membrane electrodes. A good result was obtained with tetrabenzyl pyrophosphate, and the electrode based on this ionophore and bis(1-butylpentyl) adipate as a solvent mediator in a poly(vinyl chloride) membrane matrix exhibited a near-Nernstian response to Pb2+ in the concentration range of 1x10-5-1x10-2 M with a slope of 28.7mV per concentration decade in a solution containing 0.1 M Mg(NO3)2. The limit of detection was 3x10-6 M. The selectivity of this electrode to other metal cations was comparable to the best case in many Pb2+-selective electrodes so far developed. Addition of potassium tetrakis (p-chlorophenyl)borate (40 mol% relative to tetrabenzyl pyrophosphate) caused a drastic change in the response slope (53.3 mV per concentration decade), probably due to the formation of PbA+, where A stands for anions present in the sample solution, and decreased significantly the electrode selectivity to other metal cations.[2]
Tetrasaccharide phosphate synthesis
The synthesis of dibenzyl 6-O-naphthylmethyl-2,3,5-tri-O-benzoyl-beta-D-galactofuranosyl-(1-->5)-2,3-di-O-benzoyl-6-O-benzyl-beta-D-galactofuranosyl-(1-->4)-3-O-benzyl-2-O-pivaloyl-alpha-l-rhamnopyranosyl-(1-->3)-2-acetamido-2-deoxy-4,6-di-O-benzoyl-alpha-D-glucopyranosyl phosphate (1), a protected form of the tetrasaccharide phosphate of the linkage region of the arabinogalactan-peptidoglycan complex in the mycobacterial cell wall, has been accomplished. Key steps include the coupling of four monosaccharide building blocks with complete stereoselectivity by glycosylations employing thioglycosides, 2'-carboxybenzyl glycosides, and glycosyl fluorides as glycosyl donors. The alpha-glycosyl phosphate linkage was also stereoselectively elaborated by reaction of a tetrasaccharide hemiacetal with tetrabenzyl pyrophosphate in the presence of a base.[3]
Alpha-phosphates of the 3,6-dideoxyhexoses
Interaction of lithium alcoholates of 2,4-di-O-benzoates of paratose and abequose with tetrabenzyl pyrophosphate gave alpha-phosphates of the 3,6-dideoxyhexoses, further converted into the corresponding cytidine-5'-diphosphate derivatives. These synthetic nucleotides were shown to participate in the biosynthesis of the O-specific polysaccharides for Salmonella typhimurium and S. nitra.[4]
Phosphorylation of the 1,4-diol
A glycophospholipid consisting in a derivative of N,N'-acylated and bisphosphorylated 2,3-dideoxy-2,3-diamino-D-glucose, bearing a 6-aminocaproyl side chain as spacer arm at carbon 6 (PPDm2-B), has been synthesized and its effect on murine macrophages evaluated. The synthesis started from 2,3-diamino-D-glucose, which was best obtained from glucosamine essentially by known procedures, since attempts to use another known precursor (3-nitroglycoside) led to unexpected results. Selective N-acylation was performed with the hydroxysuccinimide ester of (D)-3-benzyloxymyritic acid followed by esterification of the sole primary hydroxyl function by 6-azidocaproylchloride and phosphorylation of the resulting 1,4-diol by treatment with tetrabenzyl pyrophosphate. Hydrogenation on a Pd on carbon catalyst permitted the isolation of 6-(6-aminohexanoyl)-2,3-dideoxy-2,3-di-[(R)-3-hydroxy-tetradecanamido ] -alpha-D-glucopyranose 1,4-diphosphate (PPDm2-B). In mouse macrophages, PPDm2-B enhanced the lipopolysaccharide (LPS)-dependent secretion of tumor necrosis factor alpha (TNF-alpha), and inhibited the LPS-induced desensitization of these cells. The data suggest that PPDm2-B interacts in a serum-independent way with an LPS receptor different from CD14, and involved in endotoxin tolerance. Binding studies of a fluorescent derivative of PPDm2-B indicated that the expression of this unknown receptor is down-regulated during in vitro culture of the cells. Owing to its spacer arm, PPDm2-B could thus be a promising tool for future studies of this receptor.[5]
References
1.Hodson LE, Joseph Tholath P, Jacobs L, et al. Mild and Chemoselective Triethylsilane-Mediated Debenzylation for Phosphate Synthesis. Org Lett. 2025;27(1):246-251. doi:10.1021/acs.orglett.4c04258
2.Xu D, Katsu T. Tetrabenzyl pyrophosphate as a new class of neutral carrier responsive to lead ion. Talanta. 2000;51(2):365-371. doi:10.1016/s0039-9140(99)00286-6
3.Lee YJ, Fulse DB, Kim KS. Synthesis of a tetrasaccharide phosphate from the linkage region of the arabinogalactan-peptidoglycan complex in the mycobacterial cell wall. Carbohydr Res. 2008;343(10-11):1574-1584. doi:10.1016/j.carres.2008.05.009
4.Utkina NS, Danilov LL, Druzhinina TN, Shibaev VN. Sintez tsitidindifosfat-3,6-didezoksigeksoz--donorov glikozil'nykh ostatkov pri biosinteze O-spetsificheskikh polisakharidov salmonell serogrupp A, B i C [Synthesis of cytidine diphosphate-3,6-dideoxyhexoses--donors of glycosyl residues in the biosynthesis of O-specific polysaccharides of Salmonella of serogroups A, B and C]. Bioorg Khim. 1989;15(10):1375-1383.
5.Charon D, Mondange M, Pons JF, Blay KL, Chaby R. Synthesis and in vitro activities of a spacer-containing glycophospholipid ligand of a lipopolysaccharide receptor involved in endotoxin tolerance. Bioorg Med Chem. 1998;6(6):755-765. doi:10.1016/s0968-0896(98)00027-3
Lastest Price from Tetrabenzyl pyrophosphate manufacturers
US $1.10-9.90/kg2025-08-18
- CAS:
- 990-91-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg

US $1.00/kg2025-04-21
- CAS:
- 990-91-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt