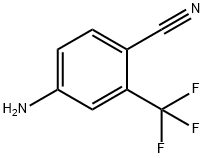
Application research of 4-amino-2-(trifluoromethyl)benzonitrile
Introduction
Bicalutamide is an oral, once-daily nonsteroidal antiandrogen. Its efficacy in localised or locally advanced prostate cancer is currently being investigated as part of the Early Prostate Cancer (EPC) programme.[1] With its high growth and good clinical performance, bicalutamide gain more and more sharing in the global prostate cancer medicine market. The demand of bicalutamide intermediate also increase quickly. Such as there should be a broad market prospect for the research of bicalutamide intermediate. 4-Amino-2-(trifluoromethyl)benzonitrile (Figure 1) is one of the most important intermediate of bicalutamide. [2]

4-Amino-2-(trifluoromethyl)benzonitrile Synthesis
In recent years, there is greater progress on the synthesis of 4-amino-2-(trifluoromethyl)benzonitrile in china. But they share a disadvantage that increases the risk of operation: using highly toxic reagent cuprons cyanide. In Xie’s paper,research was carried on the existing synthesis technology4-amino-2-(trifluoromethyl)benzonitrile. Three synthesis routes are designed.(1)4-amin0-2-(trifluoromethyl)benzonitrile is obtained from o-bromo(trifluoromethyl)benzene via nitration, cyanidation and reduction reaction. The yield is 52.2% and the purity is97%. lt is not suitable for industrialized production because of the low yield and purity. (2)4-amino-2-(trifluoromethyl)benzonitrile is obtained from o-bromo(trifluoromethyl)benzene via nitration, reduction reaction and cyanidation. The yield is up to 72.7% and the purity is 98%. The yield and purity is improved, but the cost is increased because of using Pd/C as the catalyst. It is also not suitable for industrialized production. (3)4-amino-2-(trifluoromethyl)benzonitrile is obtained from m-fluoro(trifluoromethyl)benzene via bromination, grignard reaction. cyanidation and amination reaction. The yield is up to 45.7% and the purity is 99%. All the reagents are common and cheap,which the whole cost is low. This synthesis routes is suitable for industrialized production.[2]
Modify the interface of perovskite/Spiro-OMeTAD
Flexible perovskite solar cells (f-PSCs) show unique charm in the electronics industry due to their mechanical flexibility, portability, and compatibility with curved surfaces. However, severe interfacial defects and residual tensile strain remain pivotal limitations to their performance and stability. Here, a novel strategy using 4-amino-2-(trifluoromethyl) benzonitrile (ATMB) with multiple functional groups (-NH2, -CF3, and -C≡N) is proposed to modify the interface of perovskite/Spiro-OMeTAD, realizing significant improvements in both the efficiency and stability of PSCs. The comprehensive defect passivation effects of 4-amino-2-(trifluoromethyl) benzonitrile result in a great reduction of defect density on the surface and grain boundaries of perovskite films. Moreover, the introduction of 4-amino-2-(trifluoromethyl) benzonitrile as a top interface layer reduces the Young's modulus of perovskite films and then releases the residual stress. Furthermore, ATMB modification induces an upshift of the valence band of the perovskite, facilitating hole extraction. Consequently, the rigid PSC attained a best PCE of 22.46%, and the f-PSC achieved a best PCE of 21.42% with 4-amino-2-(trifluoromethyl) benzonitrile modification, significantly exceeding the PCEs of 20.32% and 19.01% of the control devices. Furthermore, combined with phytic acid (PA)-doped SnO2, PCEs of 23.04% and 21.66% were obtained for rigid and flexible PSCs, respectively. The humidity stability, light stability, and mechanical flexibility of the devices were obviously increased.[3]
1,3,5-Triazine derivatives synthesis
4-Amino-2-(trifluoromethyl)benzonitrile is also a useful pharmacophore found in the anticancer drug bicalutamide as a structural unit. A literature survey revealed that piperazines and substituted piperazines are an important family of heterocyclic compounds attracting significant interest in medicinal chemistry. A series of 1,3,5-triazine derivatives that contain 4-amino-2-(trifluoromethyl)benzonitrile, 8-hydroxyquinoline, and different piperazines as substituents at the carbon atoms of the triazine ring have been synthesized by a simple and efficient synthetic protocol. The chemical structures of the compounds were elucidated with the aid of IR, 1H-NMR and 13C-NMR spectroscopy, and elemental analysis. The antimicrobial activity of the compounds was tested against seven bacteria (Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 619, Escherichia coli MTCC 739, Pseudomonas aeruginosa MTCC 741, Klebsiella pneumoniae MTCC 109, Salmonella typhi MTCC 733, Proteus vulgaris MTCC 1771) and four fungi (Aspergillus niger MTCC 282, Aspergillus fumigatus MTCC 343, Aspergillus clavatus MTCC 1323, Candida albicans MTCC 183). The results indicate that some of the novel s-triazines have noteworthy activity in minimum inhibitory concentration as well as agar diffusion tests.[4]
S-23 and its metabolites
(S)-N-(4-Cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide, commonly known as S-23, is an arylpropionamide SARM that has become available online relatively recently. It has been investigated in animal models for use as a male hormonal contraceptive. S-23 is structurally very similar to the well known and well-studied SARMs S4 (andarine) and S22 (ostarine), sharing the same core arylpropionamide structure, with differences only in the substituent groups of the benzene rings.The phase I and II metabolism of S-23 has been investigated in vitro, with equine liver microsomes7 and homogonised horse liver systems, as well as with human liver microsomes. The observed metabolic pathways were similar to the other arylpropionamide SARMs. The most abundant equine phase I metabolites of S-23 observed in vitro were two corresponding amide hydrolysis metabolites,4-amino-2-(trifluoromethyl)benzonitrile and 3-(4-chloro-3-fluorophenoxy)-2-hydroxy-2-methylpropionic acid, as well as a hydroxylated 4-amino-2-(trifluoromethyl)benzonitrile, the sulphate conjugate of which was the most abundant metabolite observed in the phase II experiments. The purpose of this study was to investigate the detection of S-23 and its phase I metabolites in equine urine and plasma following a multiple dose oral administration to two Thoroughbred racehorses. Liquid chromatography-high resolution mass spectrometry was used for metabolite identification, and liquid chromatography-tandem mass spectrometry was used for full sample analysis and generation of urine and plasma profiles. S-23 and seven phase I metabolites were observed in urine following enzyme hydrolysis and solvolysis. The most abundant analyte detected was the hydroxylated 4-amino-2-(trifluoromethyl)benzonitrile metabolite, which also allowed the longest duration of detection in urine from both horses, for up to 360 h following administration. The data suggest that this metabolite was likely to be highly conjugated with both sulphate and glucuronide moieties. In plasma, S-23 and two phase I metabolites were observed. S-23 was the most abundant analyte detected for both horses, allowing detection for up to 143 h post-administration. To the best of the authors' knowledge, this is the first report of S-23 and metabolites in equine urine and plasma samples.[5]
References
1. Carswell CI, Figgitt DP. Bicalutamide: in early-stage prostate cancer. Drugs. 2002;62(17):2471-2481. doi:10.2165/00003495-200262170-00006
2. Xie Y. Synthesis of 4-amino-2-(trifluoromethyl)benzonitrile as the intermediate of Bicalutamide[D].Dalian University of Technology,2013.
3. Zhao D, Li S, Zhang C, et al. Defect Control and Strain Regulation Enabled High Efficiency and Stability in Flexible Perovskite Solar Cells. ACS Appl Mater Interfaces. 2025;17(8):12961-12972. doi:10.1021/acsami.4c22642
4. Patel RV, Kumari P, Chikhalia KH. Fluorinated s-triazinyl piperazines as antimicrobial agents. Z Naturforsch C J Biosci. 2011;66(7-8):345-352. doi:10.1515/znc-2011-7-805
5. Cutler C, Viljanto M, Hincks P, Habershon-Butcher J, Scarth J, van Eenoo P. Detection of the selective androgen receptor modulator S-23 and its metabolites in equine urine and plasma following oral administration. Drug Test Anal. 2025;17(5):601-611. doi:10.1002/dta.3758
Lastest Price from 4-Amino-2-(trifluoromethyl)benzonitrile manufacturers
US $0.00-0.00/kg2025-08-29
- CAS:
- 654-70-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/Kg/Drum2025-04-21
- CAS:
- 654-70-6
- Min. Order:
- 1KG
- Purity:
- 99.5%min HPLC
- Supply Ability:
- 10 TONS