Testosterone Isocaproate: Cardiovascular Links and Subcutaneous Therapy
Testosterone Isocaproate is a synthetic esterified form of testosterone, a crucial male sex hormone responsible for various physiological functions. As an ester, this medicine is chemically modified to allow for a slower release of testosterone into the bloodstream after administration. This modification extends the hormone’s duration of action, reducing the frequency of required injections compared to unmodified testosterone. Commonly used in testosterone replacement therapy (TRT) and among bodybuilders, Testosterone Isocaproate is administered via intramuscular injection. It is part of testosterone blend formulations, combining multiple testosterone esters to achieve a more sustained release of the hormone. Similar to other forms of testosterone, Testosterone Isocaproate promotes the development and maintenance of male reproductive tissues, supports muscle and bone growth, and influences secondary sexual characteristics. While it is not typically prescribed for medical use on its own, it contributes to testosterone formulations designed to address conditions associated with low testosterone levels, such as hypogonadism. As with any hormone therapy, its use should be supervised by qualified healthcare professionals to manage potential side effects and ensure optimal therapeutic outcomes.

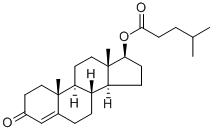
Structural Aspects and Intermolecular Energy for Testosterone Isocaproate
Testosterone Isocaproate is a cholesterol derivative and a naturally occurring anabolic steroid. It can be viewed as a derivative of the androstane group and the primary male sex hormone. It plays a major role in the development of male reproductive tissues and the maintenance of secondary male characteristics. The asymmetric unit consists of only one steroid molecule and was found to crystallise in the noncentrosymmetric P21 monoclinic space group. Ketone O1 participates in the formation of supramolecular self-assemblies, being involved in the trifurcated C-H•••O hydrogen bonding. One interaction is formed between a neighbouring six-membered B ring (C6-H6B•••O1), one binds the O1 carbonyl oxygen with a five-membered ring (C16-H16B•••O1), while the last bridges the methyl CH3 group (C19-H19A•••O1. An overall packing diagram shows the self-arrangements of steroid molecules in layers.[1]
For example, (TPro, Tphp), which differ only by the extra phenyl ring of TPhp, show a totally different orientation of the tails, as in the case of the (TAce, Testosterone Isocaproate) pair as well. It can be seen that the part of the molecules representing testosterone, the base of the ester structures, overlaps very well in all pairs. Instead, there are differences in the orientation of carbon tails. Thus, for the pair (TPro, TPhp), the C17-O2-C20-O3 torsion angle is 2.89° for TPro and −3.78° for TPhp. The angle between the planes defined by the O3-C20-O2 atoms for the pair (TPro, TPhp) is 64.93°. For the pair (TAce, TIso), the C17-O2-C20-O3 torsion angle is 3.73° for TAce and 4.93° for Testosterone Isocaproate, and the angle between the planes defined by the O3-C20-O2 atoms for the two structures is 75.03°. Due to the fact that the structures lack strong hydrogen bonds, the Coulombic energy contributes the least in the crystal packings, with similar values through all the crystals, which are in the range of −15.5 kJ/mol for TAce and −21.3 kJ/mol for Testosterone Isocaproate. Carbonyl•••hydrogen interactions were found in the Coulombic term and for the derivatives under study, the Coulombic energy has a weight between 9.7% (for TAce) and 12.6% (for TIso).
Testosterone Isocaproate and the Cardiovascular System
Recent data from the Massachusetts Male Aging Study (MMAS) have revealed an increasing incidence of hypogonadism within the aging US population. The Massachusetts Male Aging Study estimates indicate that ≈2.4 million men aged 40 to 69 suffer from hypogonadism in the United States. The Massachusetts Male Aging Study also projects ≈481 000 new cases of hypogonadism annually in US men within the same age group. The true incidence of hypogonadism among US men may be in excess of the Massachusetts Male Aging Study estimates, given the stringent criteria that were used by the authors to define hypogonadism. Testosterone Isocaproate in men reaches maximum levels at approximately age 30, after which levels steadily decline at a rate of 1% to 2% annually. Controversy exists regarding whether the decline in Testosterone Isocaproate with increasing age is a normal physiologic process or whether it is a result of chronic comorbidities and lifestyle choices. Testosterone levels are lower in patients with chronic illnesses such as end‐stage renal disease, human immunodeficiency virus, chronic obstructive pulmonary disease and several genetic conditions such as Klinefelter syndrome. It is unknown whether low testosterone in patients who are ill is the cause of their illness or whether it is caused by their disease.[2]
Given the very large population of patients in the United States who suffer from hypogonadism coupled with a projection of ≈481 000 new cases of hypogonadism annually, an extensive amount of attention has been dedicated to the interplay between Testosterone Isocaproate and various aspects of cardiovascular health and well‐being. Low endogenous bioavailable testosterone levels have been shown to be associated with higher rates of all‐cause and cardiovascular‐related mortality. Patients suffering from CAD, CHF, T2DM, and obesity have all been shown to have lower levels of endogenous testosterone compared with those in healthy controls. In addition, the severity of CAD and CHF correlates with the degree of Testosterone Isocaproate deficiency. Testosterone replacement therapy in men who suffer from hypogonadism and CAD has proven effective in increasing time to 1‐mm ST‐segment depression with exercise stress testing and causing coronary artery vasodilation. In patients with CHF, testosterone replacement therapy has been shown to significantly improve exercise tolerance while having no effect on LVEF. It is highly likely that testosterone therapy causes a shift in the skeletal muscle of CHF patients toward a higher concentration of type I muscle fibers.
Testosterone Isocaproate Therapy With Subcutaneous Injections
Testosterone Isocaproate is the main male sex hormone and is essential for the development and maintenance of male secondary sexual characteristics. Currently, testosterone therapy is indicated for men with unequivocal, organic, or pathologic androgen deficiency to alleviate symptoms and maintain secondary sexual characteristics by raising testosterone into the normal male range. In addition, testosterone therapy is used for gender-affirming (hormone) therapy for transgender men to induce masculinization (and suppress endogenous estradiol concentrations in patients with intact ovaries). In both clinical scenarios, testosterone therapy is intended to be long term. Thus, it is desirable to have various formulation options available to ensure patient satisfaction and adherence. We have come a long way since the days of Brown-Séquard, who self-administered an extract of animal testes by subcutaneous (SC) injection in 1889. It took an additional 2 years for it to be introduced into clinical medicine for the treatment of male hypogonadism with SC or intramuscular (IM) injections of short-acting ester Testosterone Isocaproate propionate, crystalline testosterone compressed into subcutaneous pellets, and oral methyltestosterone.[3]
Administration of testosterone ester via the SC route has been gaining popularity. To date, limited data suggest that SC administration of testosterone enanthate and cypionate results in stable and predictable on-treatment concentrations, has good acceptability among patients, and can be self-administered more easily than IM injections. Furthermore, localized adverse effects at the injection site are mild and transient. Although long-term studies with larger numbers of patients are needed to evaluate the safety and compliance of SC testosterone (in particular for testosterone undecanoate), clinicians should be aware of this route of testosterone administration, as it has the potential to increase patient adherence to therapy of a formulation that is relatively inexpensive and results in comparable on-treatment serum Testosterone Isocaproate concentrations.
References
[1]Turza A, Popescu V, Mare L, Borodi G. Structural Aspects and Intermolecular Energy for Some Short Testosterone Esters. Materials (Basel). 2022 Oct 17;15(20):7245. doi: 10.3390/ma15207245. PMID: 36295310; PMCID: PMC9611952.
[2]Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013 Nov 15;2(6):e000272. doi: 10.1161/JAHA.113.000272. PMID: 24242682; PMCID: PMC3886770.
[3]Figueiredo MG, Gagliano-Jucá T, Basaria S. Testosterone Therapy With Subcutaneous Injections: A Safe, Practical, and Reasonable Option. J Clin Endocrinol Metab. 2022 Feb 17;107(3):614-626. doi: 10.1210/clinem/dgab772. PMID: 34698352; PMCID: PMC9006970.
You may like
See also
Lastest Price from Testosterone isocaproate manufacturers

US $980.00/kg2025-11-24
- CAS:
- 15262-86-9
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 999

US $20.00/box2025-08-01
- CAS:
- 15262-86-9
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock