Application of Perfluorohexanes
Brief Introduction
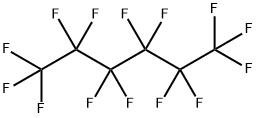
Perfluorohexane (C6F14), or tetradecafluorohexane, is a fluorocarbon and it is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. Perfluorohexanes is odorless and colorless clear liquid (figure 1) and used as a coolant, photosensitizer in fluorous biphasic singlet oxygenation and an inert reaction medium. It has a role as a radioopaque medium and a non-polar solvent [1]. It is used in the electronic cooling liquid and insulator and it plays a vital role as a reaction medium, especially for photooxidation reactions. Further, it finds application as an ultrasound contrast agent [2]. Perfluorohexane is used in one formulation of the electronic cooling liquid/insulator fluorinert for low-temperature applications due to its low boiling point of 56 °C and freezing point of −90 °C.

Figure 1 The appearance of Perfluorohexane
Application of Perfluorohexane
Perfluorohexane uses and applications include: dispersant for lubricants, mold release agents, protective coatings; reaction media for polymerizations, purification, separation processes; solvent for removal of halogenated lubricants, oils, greases; carrier for halogenated material; for quick-cooling or freezing foods; heat-transfer fluid [3].
In many applications, perfluorohexane is shown to be a good alternative to carbon tetrachloride as a non-toxic, non-ozone-depleting, inert reaction medium for bromination reactions. Yields of brominated products were nearly quantitative and the reaction work-up was easier [4].
Because it is biologically inert and chemically stable, perfluorohexane has attracted attention in medicine. Like other fluorocarbons, perfluorohexane dissolves gases, including oxygen from the air, to a higher concentration than ordinary organic solvents. This effect is attributed to the weak intermolecular forces between perfluorohexane molecules, which allows "space" for gas molecules to partition into the liquid. Animals can be submerged in a bath of perfluorohexane without drowning, as there is sufficient oxygen available in the solvent to allow respiration to continue. This effect has led to the experimental use of perfluorohexane in treating burn victims, as their lungs can be filled with either perfluorohexane vapor or in extreme cases liquid perfluorohexane, allowing breathing to continue without the problems normally seen with pulmonary edema that sometimes occur when the inside of the lungs have been burnt e.g. by inhalation of hot smoke [5].
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/Perfluorohexane#section=Substances-by-Category
[2] https://www.alfa.com/en/catalog/015812/
[3] https://www.parchem.com/chemical-supplier-distributor/Perfluorohexane-040412.aspx
[4] Suzanne M., et. al., Perfluorohexane As a Novel Reaction Medium For Bromination Reactions
Journal Synthetic Communications, An International Journal for Rapid Communication of Synthetic Organic Chemistry, Volume 25, 1995, Issue 7 Pages 1023-1026
[5] https://en.wikipedia.org/wiki/Perfluorohexane
Lastest Price from Tetradecafluorohexane manufacturers
US $0.00/kg2025-04-10
- CAS:
- 355-42-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $5.00-2.00/KG2024-10-11
- CAS:
- 355-42-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg