Application of 7-bromo-4-chloro-3-nitroquinoline
Physicochemical property
The predicted boiling point and density of 7-bromo-4-chloro-3-nitroquinoline are 387.6±37.0 °C and 1.834±0.06 g/cm3, respectively. It needs to be stored in an inert atmosphere, -20℃ refrigerator.
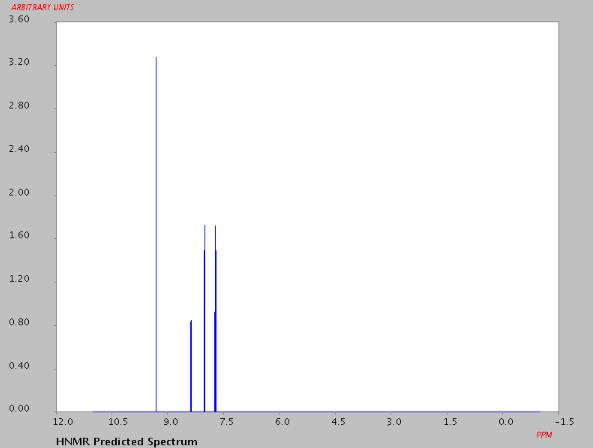
Fig.1 Predicted NMR data calculated using Advanced Chemistry Development, Inc.
Application
Novel synthetic procedures for C2 substituted imidazoquinolines
The endogenous neurotransmitter, γ-aminobutyric acid (GABA), plays a major role in neurotransmission in the mammalian brain, where it binds to ligand-gated ion channel type A receptors (GABAA). These pentameric receptors are composed of different subunits (α1-6, β1-3, γ1-3, δ, ε, π, θ, ρ1–3) and the specific assemblies thereof (receptor subtype) determine their pharmacological effects. Upon GABA binding, an anion flux is induced, which can be affected by a variety of allosteric modulators. This is of clinical importance in various ways. For example, it has been reported that sedative and anxiolytic effects are elicited by allosteric modulators (such as benzodiazepines), depending on the particular receptor subtype. A series of substituted imidazoquinolines, a structurally related chemotype to pyrazoloquinolinones, a well-known class of GABAA ligands, was prepared via two synthetic procedures and the efficiency of these procedures were compared. One method relies on classical heterocyclic synthesis, the other one aims at late-stage decoration of a truncated scaffold via direct C–H functionalization. A pharmacological evaluation disclosed that one of the synthesized derivatives showed interesting activity on a α1β3 containing receptor subtype. In this process, 7-bromo-4-chloro-3-nitroquinoline is an important intermediate [1].
Synthesis of imidazoquinolines as a novel structural class of microsomal prostaglandin E2 synthase-1 inhibitors
Prostaglandin E2 (PGE2) is well known as an important lipid mediator with broad range of biological activities in various cells and tissues, and is biosynthesized by sequential conversion of arachidonic acid with various metabolic enzymes, such as cyclooxygenase-1 (COX-1), -2 (COX-2), and PGE synthases (PGES). Among these enzymes, PGES is a terminal enzyme in the biosynthesis of PGE2 classified into three isoforms: microsomal PGES-1 (mPGES-1), -2 (mPGES-2), and cytosolic PGES (cPGES). mPGES-1, which is functionally linked to COX-2, is inducible and responsible for the release of PGE2 in response to inflammatory stimuli, such as IL-1β, TNF-α, and LPS. Indeed, a previous study has shown that PGE2 production by LPS is completely suppressed in peritoneal macrophages derived from mPGES-1 knockout mice. This allowed the use of mPGES-1 knockout mice as models of various diseases, such as collagen induced arthritis, pain hypersensitivity, and neuropathic pain. The symptoms of such diseases were significantly relieved by suppression of PGE2 production. 7-bromo-4-chloro-3-nitroquinoline is an important synthetic material in this process. The imidazoquinoline derivative 1 was found as a novel mPGES-1 inhibitor. Optimization of 1 led to the identification of the 2-chlorophenyl group at the C(2)-position and the quinolone structure at the C(4)-position. Compound 33, the most potent synthesized compound, showed excellent mPGES-1 inhibition (IC50 = 9.1 nM) with high selectivity (>1000-fold) over both COX-1 and COX-2. 7-bromo-4-chloro-3-nitroquinoline plays an important role in the synthesis of imidazoquinolines derivatives [2].
Preparation of immunoconjugate derivatives for use as HER2 modulators
It is now well appreciated that tumor growth necessitates the acquisition of mutations that facilitate immune evasion. Even so, tumorigenesis results in the accumulation of mutated antigens, or neoantigens, that are readily recognized by the host immune system following ex vivo stimulation. Why and how the immune system fails to recognize neoantigens are beginning to be elucidated. Groundbreaking studies by Carmi et al. have indicated that immune ignorance can be overcome by delivering neoantigens to activated dendritic cells via antibody-tumor immune complexes. In these studies, simultaneous delivery of tumor binding antibodies and dendritic cell adjuvants via intratumoral injections resulted in robust anti-tumor immunity. New compositions and methods for the delivery of antibodies and dendritic cell adjuvants are needed in order to reach inaccessible tumors and/or to expand treatment options for cancer patients and other subjects. 7-bromo-4-chloro-3-nitroquinoline is an important synthetic material in this process. The invention provides an immunoconjugate of formula (I) or (II). Antibody-adjuvant immunoconjugates of the invention, comprising an antibody construct that has an antigen binding domain that binds human epidermal growth factor receptor 2 ("HER2") linked to one or more adjuvants, demonstrate superior pharmacological properties over conventional antibody conjugates. The invention further provides compositions comprising and methods oftreating cancer with the immunoconjugate. 7-bromo-4-chloro-3-nitroquinoline plays an important role in the synthesis of imidazoquinolines derivatives [3].
References
[1] Draskovits M, Catorci D, Wimmer L, et al. Novel synthetic procedures for C2 substituted imidazoquinolines as ligands for the α/β-interface of the GABAA-receptor[J]. Monatshefte für Chemie-Chemical Monthly, 2022: 1-14.
[2] Shiro T, Takahashi H, Kakiguchi K, et al. Synthesis and SAR study of imidazoquinolines as a novel structural class of microsomal prostaglandin E2 synthase-1 inhibitors[J]. Bioorganic & medicinal chemistry letters, 2012, 22(1): 285-288.
[3] Ackerman SE, Alonso Michael N, Dornan D, et al. Preparation of immunoconjugate derivatives for use as HER2 modulators[P]. PCT Int. Appl., 2020, WO 2020190731.
You may like
Related articles And Qustion
See also
Lastest Price from 7-bromo-4-chloro-3-nitroquinoline manufacturers

US $1.00/g2020-01-13
- CAS:
- 723280-98-6
- Min. Order:
- 1g
- Purity:
- 99.0%
- Supply Ability:
- 100 kg

![1256544-32-7 Benzo[b]naphtho[2,3-d]furan, 3-bromo-; 3-Bromonaphtho[2,3-b]benzofuran; Application](/NewsImg/2022-12-24/6380749888624973528918087.jpg)