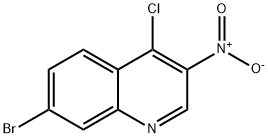
7-Bromo-4-chloro-3-nitroquinoline: Pharmaceutical Synthesis Applications and Synthesis Method
General Description
7-Bromo-4-chloro-3-nitroquinoline is crucial in pharmaceutical synthesis applications, serving as a key component in the development of NLRP3 modulators and sodium channel inhibitors. Derived compounds show promise in treating proliferative disorders like cancer and pain disorders. The synthesis method involves a multistep procedure resulting in the production of 7-Bromo-4-chloro-3-nitroquinoline, which exhibits immunomodulatory properties and holds potential in treating viral infections and cancer. This compound is synthesized to produce derivatives with inhibitory effects on TNF-α, highlighting its importance in therapeutic development for various pharmaceutical applications.

Figure 1. 7-Bromo-4-chloro-3-nitroquinoline
Pharmaceutical Synthesis Applications
NLRP3 modulators
7-Bromo-4-chloro-3-nitroquinoline, a compound utilized in the preparation of NLRP3 modulators, plays a pivotal role in combating proliferative disorders, notably cancer, within subjects. A invention outlines the compounds derived from 7-Bromo-4-chloro-3-nitroquinoline, showcasing its significance in pharmaceutical advancements. These compounds function as modulators of NLRP3, presenting a promising avenue for medicaments aimed at treating various proliferative disorders, particularly cancer, in subjects, including humans. These compounds, where R1 represents H and (un)substituted C1-6 alkyl, and R2, R3, and R5 independently denote H, halo, CN, C1-4 alkyl, C1-4 haloalkyl, among others, the potential for modulating NLRP3 is evident. Additionally, R4 is identified as 5-membered heteroaryl, while R6 encompasses (un)substituted piperidinyl, (un)substituted pyrrolidinyl, and (un)substituted pyrrolidinylmethyl. The preparation of example compound II, derived from 7-Bromo-4-chloro-3-nitroquinoline, involves a multistep procedure detailed within the invention. Notably, compound II was subject to evaluation for its NLRP3 modulatory activity, revealing an EC50 value of 10μM. This assessment underscores the efficacy and potential therapeutic significance of compounds derived from 7-Bromo-4-chloro-3-nitroquinoline in the modulation of NLRP3, thus offering promising prospects in the treatment of proliferative disorders, notably cancer, within subjects. 1
Sodium channel inhibitors
Bicyclic sulfonamide compounds derived from 7-Bromo-4-chloro-3-nitroquinoline serve as potent inhibitors of voltage-gated sodium channels, specifically Nav1.7. These compounds hold significant promise in the treatment of various disorders associated with sodium channel dysregulation, particularly pain disorders. Bicyclic sulfonamide compounds , along with their pharmaceutically acceptable salts, demonstrate remarkable efficacy in inhibiting sodium channels, which play a crucial role in the transmission of pain signals. In practical terms, Bicyclic sulfonamide compounds and related molecules offer a novel avenue for pharmaceutical intervention in pain management. By selectively targeting Nav1.7, these compounds mitigate the transmission of pain signals, offering potential relief to patients suffering from a range of pain disorders. The structural versatility of Bicyclic sulfonamide compounds allows for customization to optimize pharmacological properties. By varying substituents on ring A (including pyridine, pyrimidine, and pyridazine) and R1 and R2 groups (comprising aryl and heteroaryl moieties), researchers can fine-tune the compounds' activity and selectivity. Bicyclic sulfonamide compounds synthesized through a multistep procedure, has been rigorously evaluated for its sodium channel inhibitory activity. Notably, it exhibits impressive IC50 values of 0.0547 μM and > 10.0 μM against Nav1.7 and Nav1.5, respectively, underscoring its potential as a therapeutic agent for pain management. 2
Synthesis Method
The synthesis of 7-Bromo-4-chloro-3-nitroquinoline involves two main steps. Initially, 7-bromo[1,5]naphthyridin-4-ol undergoes a reaction with fuming nitric acid under reflux conditions for 3 hours. Following cooling and neutralization, the resulting precipitate is filtered, washed, and dried, leading to the formation of 7-bromo-3-nitro[1,5]naphthyridin-4-ol, which appears as a yellow crystalline solid. In the subsequent step, 7-Bromo-3-nitroquinolin-4-ol is suspended in POCl3 and heated under a nitrogen atmosphere. Upon dissolution, the reaction mixture is cooled, filtered, and the resulting solids are washed. This is followed by partitioning with CH2Cl2 and 2M Na2CO3. The organic layer is then separated, washed, dried, and concentrated, ultimately yielding 7-bromo-4-chloro-3-nitroquinoline as a beige solid. The resultant compound, 7-Bromo-4-chloro-3-nitroquinoline, exhibits potential as an immunomodulator of cytokine biosynthesis and finds applications in the treatment of viral infections and cancer. It is synthesized as part of a multi-step process aimed at producing imidazoquinoline and imidazonaphthyridine derivatives, which have demonstrated significant inhibitory effects on TNF-α in rat models. These derivatives hold promise for various pharmaceutical applications, showcasing their potential importance in therapeutic development. 3
Reference
1. Watterson SH, Gong H, Williams K. Imidazoquinolinamines as NLRP3 modulators and their preparation. 2021; Patent Number: WO2021142203.
2. Weiss M, Dimauro EF, Dineen T. Bicyclic sulfonamide compounds as sodium channel inhibitors and their preparation. 2014; Patent Number: WO2014201173.
3. Merrill BA, Haraldson CA, KshirsagarTushar A, Niwas S. Preparation of imidazoquinoline and imidazonaphthyridine derivatives as immunomodulators of cytokine biosynthesis. 2005; Patent Number: WO2005123080.
Related articles And Qustion
Lastest Price from 7-bromo-4-chloro-3-nitroquinoline manufacturers

US $1.00/g2020-01-13
- CAS:
- 723280-98-6
- Min. Order:
- 1g
- Purity:
- 99.0%
- Supply Ability:
- 100 kg