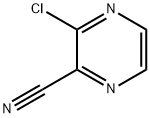
Unveiling the Potential of 3-Chloropyrazine-2-carbonitrile in Modern Chemistry
3-Chloropyrazine-2-carbonitrile is a white solid. Its molecular structure contains a chlorine atom and a cyano group, and it can participate in a variety of nucleophilic substitution reactions.
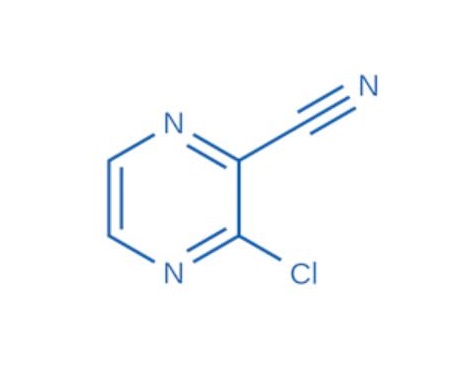
Application
Synthesis of Novel Pyrazinamide Derivatives Based on 3-Chloropyrazine-2-carboxamide
The starting compound 3-chloropyrazine-2-carboxamide was prepared via partial hydrolysis of the nitrile group of 3-chloropyrazine-2-carbonitrile (Fluorochem, Co., Hadfield, Derbyshire, UK). A mixture of concentrated (30%) hydrogen peroxide (29 mL) and water (195 mL) was prepared and alkalinized with an 8% (w/v) solution of sodium hydroxide to obtain a solution with pH 9. The carbonitrile (104 mmol) was then added portionwise into the heated (50 °C) mixture over a period of 30 min. The whole mass was stirred for an additional 2.5 h at 55 °C while the pH was periodically monitored and alternatively adjusted to the value of 9 by adding a few drops of 8% NaOH solution. The reaction mixture was cooled in a fridge to initiate crystallization. The crude product was recrystallized from ethanol. The yield of this reaction was approximately 80%.
Hazard Classes and Categories
Acute Tox. 4 (33.3%)
Acute Tox. 4 (33.3%)
Skin Irrit. 2 (100%)
Eye Irrit. 2A (100%)
Acute Tox. 4 (33.3%)
STOT SE 3 (100%)
Storage
3-Chloropyrazine-2-carbonitrile should be storaged under inert gas (nitrogen or Argon) at 2-8°C.
You may like
Related articles And Qustion
Lastest Price from 3-Chloropyrazine-2-carbonitrile manufacturers
US $0.00-0.00/mg2025-04-18
- CAS:
- 55557-52-3
- Min. Order:
- 10mg
- Purity:
- 98
- Supply Ability:
- 10000000

US $0.00-0.00/KG2025-04-04
- CAS:
- 55557-52-3
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton