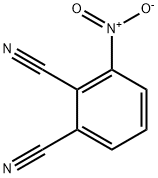
Application of 3-nitrophthalonitrile
Introduction
The organic dye and pigment industry is an important branch of the chemical industry, and its products are used in many aspects of human life and production. Among them, 3-nitrophthalonitrile can be used to synthesize a variety of important organic dyes and pigment intermediates and is a very important chemical raw material.

Picture 1 3-nitrophthalonitrile
Solubility and solution thermodynamics of 3-Nitrophthalonitrile
In this investigation, using the static equilibrium method, the solubility of 3-nitrophthalonitrile in 12 pure solvents, namely, n-propanol, methanol, ethanol, isopropanol, ethylene glycol (EG), acetone, toluene, N-methyl-pyrrolidone (NMP), n-butanol, ethyl acetate, isobutanol, and water, was investigated through experiment over a temperature range of 278.15-323.15 K under ambient pressure p = 101.2 kPa[1]. The mole fraction solubility of 3-nitrophthalonitrile in above solvents increased as the temperature increased and had the following order in different solvents: NMP > acetone > ethyl acetate > EG > methanol > ethanol > toluene > n-propanol > isopropanol > n-butanol > isobutanol > water. The solubility was correlated using the Wilson model, lambda h equation, modified Apelblat equation, and NRTL model. Results indicated that the maximum values of root-mean-square deviation and relative average deviation were 11.75 x 10(-4) and 9.26 x 10(-2), respectively. The relative average deviation values achieved with the modified Apelblat equation were lower than those with the other equations for a given solvent. Additionally, the mixing properties and reduced excess enthalpy and activity coefficient under infinitesimal concentration were derived.
The availability of 3-nitrophthalonitrile as a synthetic precursor of general utility provides a means by which many direct struture-property comparisons can be made with derivatives of 4-nitrophtjalonitrile (commercially available). The oldest reported synthesis of 3-nitrophthalonitrile is described in a patent for a one-pot, multi-step synthesis[2]. That work described the conversion of 3-nitrophthalic anhydride to the diamide derivative followed by dehydration to 3-nitrophthalonitrile using phosphorous oxychloride, some researchers developed a step-wise method which consists of preparing the phthalimide, converting it to the phthalimide, and dchydrating to the phthalonitrile. Using this procedure, they have achieved an efficient yield (>80%) and presenting a detailed characterization of 3-nitrophthalonitrile.
Application
To simply and safely obtain a corresponding 3-alkoxyphthalonitrile in high yield and high purity by reacting 3-nitrophthalonitrile with an alcohol. SOLUTION: A basic catalyst (e.g. sodium hydrate) is dispersed into a mixture of an alcohol of the formula ROH (in R, C bonded to O is a secondary or tertiary >=4C alkyl or >=5C cycloalkyl) with an aprotic polar solvent (e.g. DMF) at <=25 deg.C while retaining a state not substantially generating hydrogen. Then, 3-nitrophthalonitrile is added thereto at <=25 deg.C to provide the objective phthalonitrile compound expressed by the formula. The phthalonitrile compound is useful as a synthetic intermediate for a phthalocyanine compound used as a light recording medium, a near-infrared ray absorbing filter, an OPC, etc., for electrophotography[3].
To obtain the title derivative suitable for electronic materials, polymeric coloring matter, polymeric semiconductors, polymeric complex catalysts, etc., by substitution reaction between a polymer made up of specific recurring unit and nitrophthalonitrile. CONSTITUTION:The objective derivative made up of recurring unit of formula III ((y) is natural number; y/x : 0.1-1.0) can be obtained by substitution reaction between (A) a polymer made up of recurring unit of formula I (formula II is an expression of vinyl alcohol unit residue of PVA or its derivative; (x) is natural number) and (B) 3-nitrophthalonitrile[4].
The invention provided an alpha-(8-quinoly) single substituted zinc phthalocyanine and a preparation method thereof, with the molecular formula of C41H21N9OZn[5]; the preparation method is as follows: 8-hydroxyl quinoly and 3- nitrophthalonitrile are adopted as the original substances to synthesize 3-(8- quinoly) phthalonitrile; then 3-(8- quinoly) phthalonitrile, phthalonitrile, and the corresponding zinc salt are adopted as the original substances to synthesize a coordination compound of Phthalocyanine-zinc under the DBU catalysis, and then the coordination compound of Phthalocyanine-zinc is separated to obtain the final product. The coordination compound provided by the invention has a single structure without any isomer, and is characterized in defined structure and easy separation. The coordination compound can be applied in the high-tech fields such as the preparation of novel anti-cancer drugs.
Reference
1 Wang, Jian. Solubility and Solution Thermodynamics of 3-Nitrophthalonitrile in 12 Neat Solvents at Temperatures from 278.15 to 323.15 K,
2 R. D., & Snow, A. W. (1995). Synthesis of 3-nitrophthalonitrile and tetra-α-substituted phthalocyanines. Journal of Heterocyclic Chemistry, 32(2), 495–498.
3 PRODUCTION OF PHTHLONITRILE COMPOUND. JP3366508B2 (A) ? 2003-01-14 ? YAMAMOTO CHEMICALS INC POLYVINYL ALCOHOL DERIVATIVE AND ITS PRODUCTION
4 POLYVINYL ALCOHOL DERIVATIVE AND ITS PRODUCTION. JPH04103603A ? 1992-04-06 ? TOURE RISAACHI SENTAA KK Earliest priority: 1990-08-23 ? Earliest publication: 1992-04-06
5 Alpha-(8-quinolineoxy)mono-substituted zinc phthalocyanine and preparation method thereof. CN101260110A
See also
Lastest Price from 3-Nitrophthalonitrile manufacturers

US $2.00/kg2025-04-21
- CAS:
- 51762-67-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $9.90/KG2025-04-21
- CAS:
- 51762-67-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt