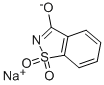
Use and preparation of Saccharin sodium
About 150 years ago, saccharin sodium was discovered by accident. It has been used as a sugar substitute to sweeten food and drinks.
Several animal studies decades ago linked saccharin sodium to possible health problems, leading to a decline in the substance's popularity. However, later studies in humans did not confirm a link to cancer.
Saccharin sodium is a kind of food additive and has no nutritional value to human body. When eating more, it will affect the normal secretion of digestive enzymes, reduce the absorption capacity of the small intestine, so that appetite loss. Many countries limit the use of sodium saccharin in food processing. In the production and management activities, a small number of enterprises in order to unilaterally pursue the sweetness and color of products or extend the shelf life of products, illegal excessive use of saccharin sodium and other food additives, posing a potential threat to human health.

Sodium saccharin is the salt most frequently used in formulating soluble forms of this sweetening agent. It can be used in toothpaste, mouthwash, diet soft drinks, syrups, baked goods, ice cream, and other sweet foods and drinks. While it is certainly most famously used in food products, sodium saccharin is also used in the chemical and agricultural industries as an aid in the production of herbicides and pesticides. It is also used as part of a solution used to coat metals, such as gold and nickel.
Major application of Sodium saccharine is the food industry as an additive in different products. It is used as a low calorie sweetener and stabilizer in a variety of food and drinks. In bakeries it is used to sweeten baked goods, breads, cookies and muffins. Due to its rapidly dissolving nature in water, it is used as an artificial sweetener in carbonated beverages and sodas.
Preparation of saccharin sodium
Adding anhydrous toluene gradually to a chlorosulfonated pan of chlorosulfonic acid, low temperature reaction, and after 3 h reaction, reaction, cooling, the complete chlorosulfonic acid decomposition, acid, and then the sulfonyl chloride to wash oil synthesis, in 15 ~ 20 ℃ refrigerated 12 h, filter out the para isomer crystallization, the liquid neighbors toluene sulfonyl chloride.
In the ammoniation pot in advance into ammonia water, add o-toluene sulfonyl chloride, reaction at 60℃ 2h, cooling, filtering, filter cake by activated carbon decolorization, in the refining pot respectively with hydrochloric acid and sodium hydroxide solution refined, o-toluene sulfonamide.
To adjacent toluene sulfonamide, add water and liquid sodium hydroxide oxide, potassium permanganate in 25 ~ 35 ℃ will be split into, and finish, 7 h thermal reaction, cooled to 25 ℃, slowly add sodium sulfite solution to oxidation solution is colorless, filtering, containing manganese dioxide filter cake washing to dry, merge the filtrate, with dilute hydrochloric acid to pH 3, precipitation is not oxide, After filtration, concentrated hydrochloric acid was added to the filtrate to precipitate completely. After filtration, the filter cake was washed with slightly acidic water, and finally insoluble saccharin was obtained.
Insoluble saccharin and sodium bicarbonate were alternately put into the neutralizing pot with water, and the reaction was dissolved by heating. The reaction solution was adjusted to neutral when the reaction temperature reached 70℃, and then filtered while hot. The filtrate was crystallized and dried to obtain sodium saccharin finished product.
You may like
Related articles And Qustion
See also
Lastest Price from Saccharin sodium manufacturers

US $1.00/kg2025-04-21
- CAS:
- 128-44-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 128-44-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month