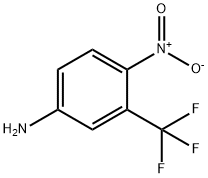
Synthesis and application of 4-Nitro-3-trifluoromethyl aniline
General description
4-Nitro-3-trifluoromethyl aniline is an organic intermediate, which can be used to prepare 4-bromo-2-nitrotrifluorotoluene, an intermediate of medicine and optical waveguide materials, and flutamide, a non steroidal anti androgen drug.
Synthetic routes
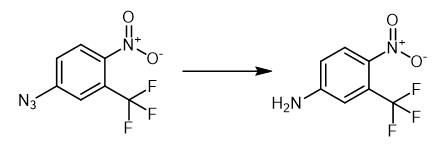
Fig. 2 The synthetic of 4-Nitro-3-trifluoromethyl aniline.
The thiazolium salt 3 (3.30 mg, 0.0122 mmol) was added to a solution of 1-azido-4-nitrobenzene (0.122 mmol) and t-BuOH (18.0 mg, 0.244 mmol) in degassed THF (0.50 mL) under argon at r.t. The resulting suspension was stirred for 5 min, and then NaOt-Bu (23.4 mg, 0.244 mmol) was added in one portion, and the mixture was stirred (Table 2). Water (2 mL) was added, and the products were extracted with EtOAc (2 × 2 mL), the combined organic phase was dried over anhyd MgSO4, filtered, and concentrated in vacuo. Purification was achieved by passing the resulting residue through a short pad of silica (eluting with 50% light PE-EtOAc) unless otherwise stated. Compound: 13, Time: 12 h, Pale yellow solid (21.0 mg, 84%). (70:30 petrol-EtOAc) 0.5; mp 90-93 °C (lit., 92-93 °C); IR (FTIR, CHCl3) νmax cm-1: 3535 (NH2), 3434 (NH2), 1635, 1592 (NO2), 1520 (NO2), 1119; 1H NMR (400 MHz, DMSO-6) 8.59 (d, = 2.7 Hz, 1H), 8.55 (dd, = 9.3, 2.7 Hz, 1H), 7.55 (br s, 2H), 7.32 (d, = 9.3 Hz, 1H); 13C NMR (100 MHz, DMSO-6) 151.8 (C), 135.0 (C), 128.7 (CH), 123.7 (J = 5.9 Hz, F3N2NaO2 [M+Na]+, 229.0195; found, 229.0195 [1].
Detection method
The objective of the present work was to study peculiarities of detection of 4-nitro-3-(trifluoromethyl)-aniline in the biological material with the use of TLC, GC-MS, and electron spectrophotometry. We have proposed the rationale for the application of acetone as an insulating agent for the extraction of 4-nitro-3-(trifluoromethyl)-aniline from the cadaveric hepatic tissue and biological fluids. It was shown that this compound is possible to separate from endogenous biomaterials on the silicagel L column (40/100 mcm). The results of the quantitative evaluation of different amounts of 4-nitro-3-(trifluoromethyl)-aniline in the cadaveric hepatic tissue, blood, plasma, and urine are presented. The proposed method makes it possible to determine a minimum of 0.12 mg of 4-nitro-3-(trifluoromethyl)-aniline in 100 g of the biological material (cadaveric hepatic tissue), 0.09 mg in 100 g of blood, 0.06 mg and 0.05 mg in 100 u of plasma and urine respectively [2].
Spectroscopic study
The Fourier transform infrared (FT-IR) and FT-Raman spectra of 4-nitro-3-(trifluoromethyl)aniline (NTFA) were recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively. Utilizing the observed FT-IR and FT-Raman data, a complete vibrational assignment and analysis of the fundamental modes of the compounds was carried out. Extensive studies on the vibrational, structural, thermodynamic characteristics as well as the electronic properties of NTFA were carried out using ab initio and OFF methods. In this kind of systems, the position of the substituent group in the benzene ring as well as its electron donor-acceptor capabilities play a very important role on the molecular and electronic properties. The values of the total dipole moment (mu) and the first order hyperpolarizability (beta) were computed using B3LYP/6-311++G(d,p) and B3LYP/6-311G(d) calculations. The Mulliken's charges, the natural bonding orbital (NBO) analysis on 4-nitro-3-(trifluoromethyl)aniline, 4-nitro-3-(trichloromethyl)aniline and 4-nitro-3-tribromomethyl)aniline were carried out for various intramolecular interactions that are responsible for the stabilization of the molecule. Thermodynamic functions of the investigated molecule were also computed. The calculated HOMO-LUMO energies show that charge transfer occurs in the molecule. The influence of fluorine, amino and nitro group on the geometry of benzene and its normal modes of vibrations has also been discussed [3].
Application
As nitric oxide (NO) photodonor
The design, synthesis, photochemical properties, and biological evaluation of a novel photoactivatable bichromophoric conjugate are reported. The compound 1, [4-(4,4-difluoro-2,6-diiodo-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacen-8-yl)-N-(3-((4-nitro-3-(trifluoromethyl) phenyl) amino) propyl) butanamide] combines a 2,6-diiodo-1,3,5,7-tetramethyl BODIPY derivative as singlet oxygen (O-1(2)) photosensitizer and 4-nitro-3-(trifluoromethyl) aniline (NOPD) as nitric oxide (NO) photodonor, joined by an alkyl spacer. These two chromogenic units absorb in distinct regions of the visible spectrum, and their individual photochemical properties are conserved in the molecular conjugate. Irradiation of the bichromophoric conjugate with green light afforded O-1(2) in high quantum yields, whereas O-1(2) production was negligible with the use of blue light; under this latter condition, NO was released. Photogeneration of NO and cytotoxic O-1(2) can therefore be regulated by appropriately tuning the excitation light wavelength and intensity. Tested on melanoma cancer cells, this resulted in amplified photomortality relative to that of a structurally correlated model compound 2 [4-(4,4-difluoro-2,6-diiodo-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacen-8-yl)-N-(3-(p-tolylamino)propyl)butanamide] deprived of the NO-release capacity. The cellular uptake of 1, evaluated by confocal fluorescence microscopy, showed that the product is localized in the cytoplasm [4].
References
[1] Burnley J, Carbone G, Moses J E. Catalytic reduction of ortho-and para-azidonitrobenzenes via tert-butoxide ion mediated electron transfer[J]. Synlett, 2013, 24(05): 652-656.
[2] Shormanov V K, Andreeva Y V, Omel'chenko V A. Peculiarities of detection of 4-nitro-3-(trifluoromethyl)-aniline in the biological material[J]. Sudebno-meditsinskaia Ekspertiza, 2016, 59(3): 31-37.
[3] Saravanan S, Balachandran V, Viswanathan K. Spectroscopic investigation of 4-nitro-3-(trifluoromethyl) aniline, NBO analysis with 4-nitro-3-(trichloromethyl) aniline and 4-nitro-3-(tribromomethyl) aniline[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 121: 685-697.
[4] Fraix A, Blangetti M, Guglielmo S, et al. Light‐Tunable Generation of Singlet Oxygen and Nitric Oxide with a Bichromophoric Molecular Hybrid: a Bimodal Approach to Killing Cancer Cells[J]. ChemMedChem, 2016, 11(12): 1371-1379.
See also
Lastest Price from 4-Nitro-3-trifluoromethyl aniline manufacturers

US $10.00/kg2025-04-21
- CAS:
- 393-11-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $260.00/g2025-04-21
- CAS:
- 393-11-3
- Min. Order:
- 50g
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month