Application examples of 4-Nitrophthalonitrile
Introduction
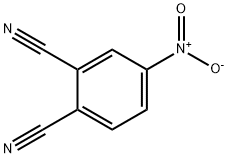
4-Nitrophthalonitrile (Figure 1) is an efficient, low toxicity, broad-spectrum pesticide intermediate, and also an intermediate of high-grade dyes. As an intermediate, 4-nitrophthalonitrile is widely used in medicine, new materials and other fields, especially in the preparation of phthalocyanine compounds, which are described as new materials in the 21st century. 4-Nitrophthalonitrile was synthesized from phthalic andydride through ammoiation, nitration,aminolysis, then it was dehydrated to produce 4-nitrophthalonitrile.The reaction is mild, the product is pure and the total yield reaches 36%.[1]

4-Nitrophthalonitrile modified electrode for L-glutathione detection
Recently, a new type of mediator was studied by us, based on 4-nitrophthalonitrile-modified carbon paste electrode. The nitrocompounds, in their oxidized state, have no electrocatalytic activity for thiol oxidation. However, when electrochemically reduced, the nitro-group is transformed into a hydroxylamino functionality. The resulting hydroxylamine can, thus, be oxidized reversibly to the corresponding nitro so compound (RNO/RNHOH couple), by α 2e−/2H+redox process, giving a stable (NC)2C6H3–NHOH/(NC)2C6H3–NO redox couple-modified electrode.In this sense, the present work explores the electrocatalytic activity of Ar–NO/Ar–NHOH redox couple from 4-nitrophthalonitrile modified electrode for L-glutathione detection.[2]
After optimizing the operational conditions, the sensor provided a linear response range for GSH from 8.0 up to 83.0 mol/L with sensitivity, detectionand quantification limits of 54 nALμmol−1, 2.7μmol/L and 8.0μmol/L, respectively. The proposed sensor presented higher sensitivity when compared to other modified electrodes described in the literature and showed a stable response for at least 100 successive determinations. The repeatability of the measurements with the same sensor and different sensors, evaluated in terms of relative standard deviation, were 4.1 and 5.0%, respectively, for n=10. The developed sensor was applied for GSH determination in yeast extract and the results were statistically the same with those obtained by the comparative method described in the literature at a confidence level of 95%.[2]
4-Nitrophthalonitrile as a proper acceptor catalyst
Photoexcitation of the electron-donor-acceptor complexes have been an effective approach to achieve radicals by triggering electron transfer. However, the catalytic version of electron-donor-acceptor complex photoactivation is quite underdeveloped comparing to the well-established utilization of electronically biased partners. In this work, utilizing 4-nitrophthalonitrile as a catalytic acceptor, the benzylic C−H bond cleavage could be achieved by photoactivation. The Giese reaction of alkylanisoles and the oxidation reaction of the benzyl alcohols were then developed efficiently with a wide scope of substrates. Moreover, this e-poor catalyst could further activate formate salts smoothly and furnish the hydrocarboxylation reaction of alkenes and alkynes using potassium formate as a carbonxylation source. A mechanism involving the catalytic photoactivation of an EDA complex and deprotonation was proposed based on the mechanistic studies.The characterization and energy profiles on the cocrystal of 4-nitrophthalonitrile and 1,3,5-trimethoxybenzene disclose that the electron transfer is highly favorable under the light irradiation. This electron acceptor catalyst can be efficiently applied in the benzylic C−H bond photoactivation by developing the Giese reaction of alkylanisoles and the oxidation of the benzyl alcohols. A broad scope of electron-rich aromatics can be tolerated and a mechanism is also proposed.Moreover, the corresponding π-anion interaction of 4-nitrophthalonitrile with potassium formate can further facilitate the hydrocarboxylation of alkenes efficiently.[3]
Phthalonitrile compounds synthesis
The compound 4-(2-((1H-benzo[d]imidazol-2-yl) thio) phenoxy) phthalonitrile was obtained from the reaction of 2-nitrophenol, 4-nitrophthalonitrile and 2-mercaptobenzimidazole. This compound was reacted with magnesium Chloride (MgCl2) to yield tetrakis-[(2-((1H-benzo[d]imidazol-2-yl) thio) phenoxy) phthalocyaninato] magnesium II. New compounds were characterized by UV-vis, 1H-NMR, 13C-NMR, FTIR and Mass spectra. Electronic spectra aggregation study of magnesium phthalocyanine compound in various concentrations and diverse solvents was performed. Photoluminescence spectra of magnesium phthalocyanine in different solvents were investigated. The biological activities of 3 and 4 compounds were investigated. The results showed that 4 had excellent antioxidant and antidiabetic activities as 75.71% and 81.83%, respectively. 3 and 4 had deoxyribonucleic acid (DNA) cleavage ability and 4 caused a double-strand fracture in plasmid DNA at 100 and 200 mg/L. Both compounds showed antimicrobial activity and also 4 was more effective against pathogenic microorganisms than 3. Photodynamic antimicrobial therapy of test compound was also more effective than without irradiation. The highest biofilm inhibition of 3 and 4 was 78.28% and 98.49% for S. aureus and also 73.95% and 91.13% for P. aeruginosa, respectively. Finally, both compounds demonstrated %100 microbial cell viability inhibition at 100 mg/L. Overall, the study suggests that both 3 and 4 have potential for further development as therapeutic agents.[4]
References
1.Yu MJ,et al.A Study On Synthesis Of 4-Nitrophthalonitrile[J].Science & Technology in Chemical Industry,2001,(02):16-18.DOI:10.16664/j.cnki.issn1008-0511.2001.02.007.
2.Lima PR, Santos WJ, Oliveira AB, Goulart MO, Kubota LT. Electrocatalytic activity of 4-nitrophthalonitrile-modified electrode for the l-glutathione detection. J Pharm Biomed Anal. 2008;47(4-5):758-764. doi:10.1016/j.jpba.2008.03.006
3.Xue T, Ma C, Liu L, Xiao C, Ni SF, Zeng R. Characterization of A π-π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis. Nat Commun. 2024;15(1):1455. Published 2024 Feb 16. doi:10.1038/s41467-024-45686-1
4.Özdemir S, Güngördü Solğun D, Giray G, Ağırtaş MS. Synthesis and biological activity, photophysical, photochemical properties of tetra substituted magnesium phthalocyanine. Photochem Photobiol Sci. 2025;24(2):277-292. doi:10.1007/s43630-025-00686-y
You may like
See also
Lastest Price from 4-Nitrophthalonitrile manufacturers

US $10.00/kg2025-04-21
- CAS:
- 31643-49-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton

US $76.74/Kg/Drum2025-04-21
- CAS:
- 31643-49-9
- Min. Order:
- 25Kg/Drum
- Purity:
- 99.50%HPLC
- Supply Ability:
- 3tons/month