2,3,4-Trimethoxybenzaldehyde: Properties and Anti-Candida Activity
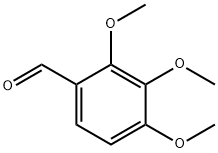
2,3,4-Trimethoxybenzaldehyde is an important aromatic aldehydes, the chemical formula is C₁₀H₁₂O₄, molecular weight 208.20, the molecular structure of the benzene ring of the 2, 3, 4 positions are replaced by a methoxy (-OCH₃), the para position (1 position) connected aldehyde (-CHO). In the molecular structure, the benzene ring is substituted with a methoxy group (-OCH₃) in the 2, 3, and 4 positions respectively, and an aldehyde group (-CHO) is attached to the opposite position (1 position). This multimethoxy substitution structure gives it unique chemical activity and application value, widely used in medicine, spices, dyes and other fields, is an indispensable intermediate in organic synthesis. In terms of physical and chemical properties, 2,3,4-trimethoxybenzaldehyde usually appears as white to light yellow needle-like crystals or powder, with a weak aromatic odor, easily soluble in organic solvents such as ethanol, ether, chloroform, etc., with low solubility in water (solubility of about 0.5g/L at 25℃). Its melting point is 74-77 ° C, boiling point is 163-165 ° C (1.33kPa), stable chemical properties at room temperature and normal pressure, but if long-term exposure to high temperatures, bright light or strong oxidants in the environment, the aldehyde oxidation, hydrolysis of methoxy and other reactions may occur. The aldehyde group in the molecule has typical aldehydes reaction properties, and can undergo nucleophilic addition, condensation, redox and other reactions; and the electron-donating effect of the three methoxyl groups can enhance the benzene ring electron cloud density, further enhance the reaction activity of the aldehyde group, and provide a basis for the subsequent synthesis of derivative compounds.

Anti-Candida properties of asaronaldehyde of Acorus gramineus rhizome and three structural isomers
Asaronaldehyde is an active component of Acorus gramineus rhizome. This study aims to evaluate the anti-Candida efficacy of asaronaldehyde and its three structural isomers, namely, 2,3,4-trimethoxybenzaldehyde, 3,4,5-trimethoxybenzaldehyde, and 2,4,6- trimethoxybenzaldehyde. However, structurally related compounds differ in their bioactivities, and the difference in the conformation and position of the functional group(s) determines their binding affinity to target molecules and clinical efficacy. To our knowledge, the effects of asaronaldehyde and its structural isomers, namely, 2,3,4-trimethoxybenzaldehyde, 3,4,5-trimethoxybenzaldehyde, and 2,4,6-trimethoxybenzaldehyde, on the human pathogenic yeast C. albicans, have not been reported. However, alpha and beta asarones are known to exhibit anti-C. albicans activity. The compounds tested in this study, which differ only in terms of the conformation or position of methoxy groups, showed various degrees of antifungal activity. All of the compounds significantly (P = 0.0412 for 2,4,5-trimethoxybenzaldehyde, P = 0.0454 for 2,4,5-trimethoxybenzaldehyde, P = 0.0490 for 2,3,4-trimethoxybenzaldehyde, P = 0.0475 for 3,4,5-trimethoxybenzaldehyde) inhibited planktonic growth of C. albicans in a concentration-dependent manner. 2,4,6-trimethoxybenzaldehyde inhibited the growth and viability of C. albicans at 0.25 and 1 mg/mL, respectively, while 2,4,5-trimethoxybenzaldehyde, 2,3,4-trimethoxybenzaldehyde and 3,4,5-trimethoxybenzaldehyde showed MIC at 1 mg/mL.[1]
The MFC of 2,3,4-trimethoxybenzaldehyde was 2 mg/mL, whereas the MFCs for 2,4,5-trimethoxybenzaldehyde and 3,4,5-trimethoxybenzaldehyde were 8 and 4 mg/mL, respectively. Complete inhibition of Candida growth by the four compounds was observed at sub-inhibitory concentrations (P = 0.0402 for 2,4,5-trimethoxybenzaldehyde, P = 0.0410 for 2,4,5- trimethoxybenzaldehyde, P = 0.0480 for 2,3,4-trimethoxybenzaldehyde, and P = 0.0497 for 3,4,5-trimethoxybenzaldehyde). All four compounds inhibited 80–100% of the yeast to hyphal transition at sub-inhibitory concentrations (P = 0.0353 for 2,4,5-trimethoxybenzaldehyde, P = 0.0301 for 2,4,5-trimethoxybenzaldehyde, P = 0.0390 for 2,3,4-trimethoxybenzaldehyde, and P = 0.0405 for 3,4,5-trimethoxybenzaldehyde). In addition to germ tube inhibition, inhibition of budding was also recorded. A greater than 50% of reduction in morphogenesis was observed at 0.125 mg/mL for 2,3,4-trimethoxybenzaldehyde, 3,4,5-trimethoxybenzaldehyde and 2,4,6-trimethoxybenzaldehyde, while similar results were observed at 0.25 mg/mL for 2,4,5-trimethoxybenzaldehyde. Inhibiting virulence factors, e.g., dimorphism, without killing the pathogen, might avoid natural selection and thereby prevent the emergence of a drug-resistant population.
References
[1]Rajput SB, Shinde RB, Routh MM, Karuppayil SM. Anti-Candida properties of asaronaldehyde of Acorus gramineus rhizome and three structural isomers. Chin Med. 2013 Sep 8;8(1):18. doi: 10.1186/1749-8546-8-18. PMID: 24010893; PMCID: PMC3847626.
You may like
Lastest Price from 2,3,4-Trimethoxybenzaldehyde manufacturers

US $10.00/kg2025-04-21
- CAS:
- 2103-57-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton

US $0.00-0.00/KG2025-04-15
- CAS:
- 2103-57-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg