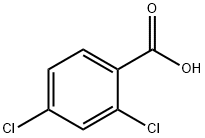
2,4-Dichlorobenzoic Acid: Sources, Decomposition, Toxicology and Biodegradation
2,4-Dichlorobenzoic acid is defined as a halogenated organic compound that exhibits nucleophilic substitution activity, with its reactivity influenced by the presence of electron-withdrawing groups which can enhance the susceptibility of the C−Cl bond to nucleophilic attack.

Natural Products Produced by the Terrestrial Cyanobacterium Fischerella ambigua
Chemical investigations into the secondary metabolite content of cyanobacteria have led to the isolation of numerous biologically active compounds. After cultivation, isolated biomass was frozen, freeze-dried, and extracted with dichloromethane (CH2Cl2), followed by methanol (MeOH). Normal-phase vacuum liquid chromatography (VLC) of the CH2Cl2 extracts, employing a step gradient from n-hexane to EtOAc, and finally to MeOH, yielded several fractions that were used for chemical (TLC, 1H NMR) and biological (antimicrobial, antiviral, ecotoxicological) screening. The culture medium of the BG-11 trial was concentrated on XAD-16 resin, and retained compounds were eluted with MeOH. Analysis of fractions originating from the cyanobacterial biomass grown in BG-11 medium led to the isolation of the new natural product ambigol C together with the known metabolites 2,4-dichlorobenzoic acid , ambigol A , and tjipanazole D .[1]
The 13C NMR spectrum of 2 contained six resonances for aromatic carbons and a quaternary carbon (168 ppm), indicating a ring substituent. The latter was thus substituted with a carboxyl group and two chlorine atoms. With these data in hand, it was evident that 2 was 2,4-dichlorobenzoic acid, a deduction that was confirmed by comparison of all data with those of an authentic sample. This is the first report of this compound to be produced in nature. The new natural products 3,5-bis(2,4-dichlorophenoxy)-2,6-dichlorophenol, a highly chlorinated aromatic compound, and 2,4-dichlorobenzoic acid were isolated from the terrestrial cyanobacterium Fischerella ambigua together with the known compounds ambigol A and tjipanazole D. All structures were secured by extensive spectroscopic analysis (1D and 2D NMR, MS, UV, IR). Ambigol C has moderate activity against Trypanosoma rhodesiense.
Decomposition Products of the Initiator Bis(2,4-dichlorobenzoyl)peroxide
During curing, 2,4-DCBP decomposes into a 2,4-dichlorobenzoyl radical, which can be further decarboxylated into a 2,4-dichlorophenyl radical, both of which are responsible for the starting of the polymerization process. These radicals give rise to 2,4-dichlorobenzoic acid (2,4-DCBA) and 1,3-dichlorobenzene as the main decomposition products, which are released during the production process. Although previous investigation was only pilot in nature and focused on the previously unknown occupational exposure to these “silicone-specific” PCBs, we had no opportunity to thoroughly investigate the main influence factors on the internal exposure of workers to the initiator 2,4-DCBP, particularly for the major decomposition products 2,4-DCBA and 1,3-dichlorobenzene. The present investigation was designed to fill this gap. Toxicological information on 2,4-Dichlorobenzoic acid is very sparse: It is a white solid known to be irritating to the skin with a melting point of 157–160° C. Apart from a median lethal dose of 830 mg/kg bw in mice for acute oral toxicity, no further information could be found. It can be assumed that incorporated 2,4-DCBA is excreted unchanged via urine after conjugation.[2]
For the structurally related 1,2-dichlorobenzene, the urinary half-life of the corresponding 3,4- and 4,5-DCK metabolites was reported to be 8.3 ± 0.47 h in humans after controlled exposure. We would expect the urinary half-lives of 3,5-DCK (and probably also 2,4- and 3,5-DCP) to be in the same range so that intervention measures for the workers could be easily and fast monitored using human biomonitoring within a few days. Also, 2,4-Dichlorobenzoic acid as the other main decomposition product of 2,4-DCBP was found in most of the workers’ urine samples with a maximum value of approximately 1.5 mg/L. Again, the highest levels were found in the “hot” areas of extrusion, processing, and wrapping/tempering. Due to the low volatility of 2,4-Dichlorobenzoic acid, it has only rarely been detected in workers not involved in the production process. As there is hardly any information on toxicology of 2,4-DCBA, these values cannot be interpreted from a toxicological point of view. There is also no information on urinary half-lives of 2,4-Dichlorobenzoic acid, but we would expect the excretion to be rapid with half-lives of a few hours.
Characterization of multiple chlorobenzoic acid-degrading organisms
The current study describes multiple bacterial strains obtained by a simple enrichment technique from pristine and contaminated sites that are able to grow with 2,4-diCBA in addition to one or more monoCBAs. The objective was to systematically examine growth, substrate spectra and potential metabolic routes as well as the taxonomic distribution of the organisms. Biodegradation of 2,4-Dichlorobenzoic acid is relevant because metabolism of PCBs would most certainly generates the acid from 2,4-substitution pattern since the common 2,3-dioxygenation attack of the standard PCB pathway is prohibitive. With the exception of the reports of van den Tweel et al., 1987,
Zaitsev and Karasevich, and
Miguez et al. describing strains NTB-1, KZ-4 and Alcaligenes denitrificans BRI 6011 respectively, no other organism has been shown to possess 2,4-Dichlorobenzoic acid catabolic phenotype. Our results show persuasive evidence for mineralization and the involvement of chlorocatechols in the pathway leading to eventual mineralization of the acid, contrary to existing reports.[3]
In order to compare the degradation competence of those strains that exhibited 2,4-diCBA phenotype, their growth characteristics in the presence of 2 mM of the acid as the sole source of energy and carbon was studied over a time period of 192 h. In batch experiments, growth was concomitant with substrate disappearance and near-stoichiometric release of chloride. Doubling times for 2,4-Dichlorobenzoic acid degradation doubled those determined for mono-substituted CBAs. Out of the six 2,4-diCBA degraders submitted for enzyme assays, significant induction of catechol 1,2-dioxygenase types I and II activities in cell-free extracts were found in four while protocatechuate 3,4-dioxygenase activity was detected in the remaining two. Activities in CBA-grown cells were 20 orders-of-magnitude higher than those grown on benzoic acid.
References
[1]Wright AD, Papendorf O, König GM. Ambigol C and 2,4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua. J Nat Prod. 2005 Mar;68(3):459-61. doi: 10.1021/np049640w. PMID: 15787461.
[2]Schettgen T, Esser A, Alt A, Randerath I, Kraus T, Ziegler P. Decomposition Products of the Initiator Bis(2,4-dichlorobenzoyl)peroxide in the Silicone Industry: Human Biomonitoring in Plasma and Urine of Workers. Environ Sci Technol. 2022 Jun 21;56(12):8518-8527. doi: 10.1021/acs.est.2c01530. Epub 2022 Jun 7. PMID: 35671459.
[3]Adebusoye SA, Miletto M. Characterization of multiple chlorobenzoic acid-degrading organisms from pristine and contaminated systems: mineralization of 2,4-dichlorobenzoic acid. Bioresour Technol. 2011 Feb;102(3):3041-8. doi: 10.1016/j.biortech.2010.10.026. Epub 2010 Oct 12. PMID: 21074990.
Lastest Price from 2,4-Dichlorobenzoic acid manufacturers

US $0.00-0.00/KG2025-06-24
- CAS:
- 50-84-0
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $1.00/KG2025-04-21
- CAS:
- 50-84-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt