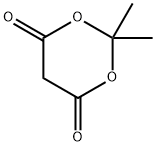
Application and toxicological of 2,2-Dimethyl-1,3-dioxane-4,6-dione
Meldrum’s acid (2,2-dimethyl-1,3-dioxane-4,6-dione; isopro-pylidene malonate) is an organic compound, discovered in 1908 by A. N. Meldrum.1 Meldrum misidentified the structure as b-lactone with carboxylic acid group at position, and the correct cyclic acylal structure was only assigned 40 years later. Meldrum’s acid has attracted considerable attention due to its high acidity (pKa = 4.97) and rigid cyclic structure. Acylated derivatives (synthetic equivalents of mixed ketenes) readily undergo alcoholysis to give b-keto esters. Alkylidene derivatives are strong electrophiles and can undergo Diels-Alder reactions with high diasteroselectivity. This versatile tool is a key intermediate for a large number of important building blocks and there remains much to discover.[1]


Figure 1 the molecular formula of 2,2-Dimethyl-1,3-dioxane-4,6-dione
Application and pharmacokinetics
For organic synthesis; Used as pharmaceutical intermediates; Accelerating Knoevenagel condensations between aldehydes and Michaelis acid in ionic liquids.[2] In the field of organic synthesis, Michaelis acid can be used as raw material to synthesize a variety of compounds with biological and pharmaceutical activities because of its important reaction characteristics.
Synthesis
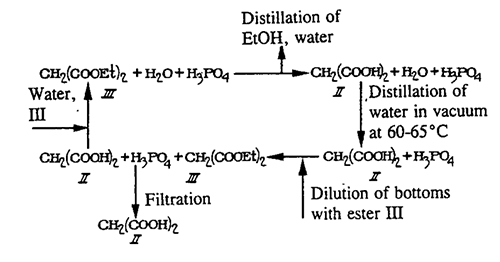
The chemistry of Meldrum’s acid has been surveyed in comprehensive reviews[2]and a micro-review. [3,4]A single review is devoted to synthetic applications of the pyrolysis of Meldrum’s acid derivatives. [5,6] Compound 1 can be classified as a cyclic acylal. Acylals are a group of organic compounds that share the functional group with the general structure R1R2C(OOCR3)2. Meldrum’s acid is usually prepared by condensation of malonic acid with acetone in acetic anhydride in the presence of sulfuric acid. Excellent yield of the product was achieved when acetic anhydride was added in a slow, controlled manner to a mixture of acetone, malonicacid and an acid catalyst.
1. The natural environment continues to be an abundant source of biologically active and structurally diverse compounds. The mounting demand for new leads in medicinal chemistry stimulates research in the field of natural product chemistry. Total syntheses of such substances not only provide sufficient amounts of material for biological studies, but also result in novel synthetic methods and strategies. Due to their unique reactivity 2,2-Dimethyl-1,3-dioxane-4,6-dione and its derivatives have proven to be valuable reagents and intermediates in the synthesis of complex organic compounds such as natural products and their analogs. The ability of acyl derivatives of 2,2-Dimethyl-1,3-dioxane-4,6-dione to generate acylketene species under pyrolysis conditions is the most fruitful field of their applications. For example, b-ketothioesters, easily accessible from reaction of thiols with 2,2-Dimethyl-1,3-dioxane-4,6-dione, can be regarded as analogs of acyl-SCoA and exploited in biomimetic syntheses of polyketide derived natural products. As demonstrated in the present review, cyclic acylals have a potential for application in stereoselective synthesis of complex organic molecules. Another direction in their chemistry is the development of novel multicomponent and domino reactions, producing variously substituted privi-leged scaffolds. These reactions, along with 2,2-Dimethyl-1,3-dioxane-4,6-dione based solid phase syntheses, are ideally suited for parallel and combinatorial processing. Parallelization techniques provide easy exploration of the chemical space around the biologically active scaffolds, enabling generation of natural product-like libraries for biological screening and SAR studies.
2. The pyrolysis of Meldrum’s acid derivatives in solution and in gas phase takes place by loss of acetone and carbon dioxide to provide ketene intermediates. In particular, methylene derivatives of ten provide methyleneketenes, which act as substrates for internal hydrogen transfer, leading to cyclization reactions. These cyclization reactions are used for the efficient preparation of a diverse range of cyclic compounds such as quinolinones, 3-hydroxythiophenes, naphthols, azepin-3(2H)-ones or pyrrolizin-3-ones, initiated respectively by 1,3-, 1,4-, 1,5-, 1,6 or 1,7-prototropic shifts.

Figure 2 Synthesis of 2,2-Dimethyl-1,3-dioxane-4,6-dione
Toxicological and Safety
If inhaled, remove the patient to fresh air., If breathing stops, perform artificial respiration. Rinse with soap and plenty of water.Wash eyes with water carefully. Never feed anything to an unconscious person., Rinse your mouth with water.
Fire extinguishing methods and extinguishing agents are water mist, alcohol resistant foam, dry powder or carbon dioxide fire extinguishing.
References
1
2.Liu Yanhang. Study on multi-component "one pot" synthesis of merrillic acid derivatives [D]. Liaoning Normal University, 2016
3. H. McNab, Chem. Soc. Rev., 1978, 7, 345–358.
4. B.-C. Chen, Heterocycles, 1991, 32, 529–597.
5. V. D. B. Bonifa ´ cio, Synlett, 1993, 1649–1650.
6. A. E.-A. M. Gaber and H. McNab, Synthesis, 2001, 2059–2074.
You may like
Related articles And Qustion
Lastest Price from 2,2-Dimethyl-1,3-dioxane-4,6-dione manufacturers

US $0.00-0.00/KG2025-09-11
- CAS:
- 2033-24-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 100KG

US $250.00/kg2025-08-01
- CAS:
- 2033-24-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 70tons