Description
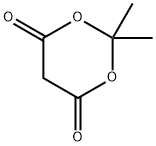
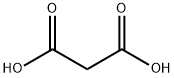
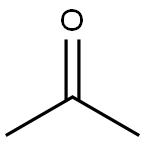
Meldrum's acid, also known as 2,2-dimethyl-1,3-dioxane-4,6-dione, is an organic compound with the formula C6H8O4. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid. Meldrum misidentified the structure as a β-lactone of β-hydroxyisopropylmalonic acid. Yet Meldrum's acid has a high acidity with a pKa of 4.97, because like ascorbic acid, deprotonation at the methylene next to the carbonyls produces a stable enolate. Because of this property Meldrum's acid like malonic acid is a reactant in Knoevenagel condensations.
Chemical Properties
The compound appears as white to beige crystals. It has a melting point of 94-95℃ (decomposition), is insoluble in water, and is soluble in ethanol and acetone.
Application
2,2-Dimethyl-1,3-dioxane-4,6-dione was used in the synthesis of:
macrocyclic β-keto lactone
4-pyridyl-substituted heterocycles
2-substituted indoles
isofraxidin.
Uses
An antimicrobial agent
Uses
Meldrum's acid serves as an intermediate in a variety of organic synthesis reactions. It is commonly used as an alternative to acyclic malonic esters and generally acts as a C3O2 synthon in organic synthesis.
Preparation
2,2-Dimethyl-1,3-dioxane-4,6-dione is usually prepared by condensation of malonic acid with acetone in acetic anhydride in the presence of sulfuric acid. Excellent yield of the product was achieved when acetic anhydride was added in a slow, controlled manner to a mixture of acetone, malonic acid and an acid catalyst.
Synthesis Reference(s)
Journal of the American Chemical Society, 70, p. 3426, 1948
DOI: 10.1021/ja01190a060
General Description
2,2-Dimethyl-1,3-dioxane-4,6-dione (Meldrum′s acid) is widely used in organic synthesis, especially for multiple C-C bond formations due to its adequate acidity (p
Ka 4.83) and steric rigidity. Knoevenagel condensation reaction between aldehydes and Meldrum′s acid are accelerated in ionic liquids.
Flammability and Explosibility
Non flammable
Purification Methods
Crystallise the dione from Me2CO/H2O. It is a synthon for the C3 malonic acid moiety. [Arnette et al. J Am Chem Soc 106 6759 1984, Bihlmayer et al. Monatsh Chem 98 564 1967, Review: McNab Chem Soc, Rev 7 345 1978, Chan & Huang Synthesis 452 1982, Beilstein 19/5 V 8.]