Application and research of 3,3'-Bicarbazole
General description
3,3'-bicarbazole is reported few. Carbazole based dimers, oligomers, and polymers have been used in OLEDs as blue, white, green, and red emitters due to high thermal, morphological, chemical and environmental stability, fine hole transport and electroluminescent properties. 3,3′-Bicarbazolyl derivatives possess superior glass forming tendency, along with low ionization potential and show good hole injection and transport properties than carbazole only materials and can be employed as the blue-light-emitting materials in OLEDs[1].
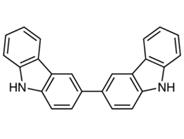

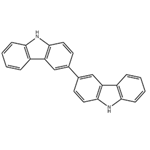
Figure1 the molecular formula of 3,3'-bicarbazole
Application and research
Liu et al.[2] showed that a conjugated polymer containing pyridine in the main chain exhibited potential application in polymeric memory materials. Solution-processed organic light-emitting diodes (OLEDs) are very attractive due to their low cost, large area display and lighting characteristics. Small molecules and polymers can be used as host materials in the solution-processed light-emitting layer.
Kim Jungwoon et al.[3]reported two small host molecules based on 3,3'-bicarbazole, which have a structural isomer relationship. 9,9'-di-4-n-butylphenyl-9 H ,9' H -3,3'-bicarbazole (BCz-nBuPh) and 9,9'-di-4-tert-butylphenyl -9 H ,9' H -3,3'-bicarbazole (BCz-tBuPh) exhibits similar optical properties in solution, but exhibits different photoluminescence in the film. Compared with the device with the BCz-nBuPh body, the solution-processed green phosphorescent OLED with the BCz-tBuPh body has the maximum current efficiency and power efficiency of 43.1 cd/A and 40.0 lm/W, respectively. Armands Ruduss et al. [4]reported a new type of 3,3'-bicarbazole-based charge transport material, mainly designed for systems containing phosphorescent iridium (III) composite emitters. A low-cost oxidative coupling reaction using FeCl3 is used to synthesize 3,3'-bicarbazole compounds. Different derivatives of 3,3'-bicarbazole have 4-ethoxyphenyl and ethyl-substituents at the 9,9'-position and (2,2-diphenyl Hydrazone) methyl- and 4-(dimethylamino)styryl-substituents. Synthetic. The obtained (2,2-diphenylhydrazone) methyl derivative exhibits a glass transition temperature sufficient for use in electronic devices. A spin coating method can be used to produce amorphous thin films with good optical quality from synthetic materials.
Researched 6,6'- and 4-ethoxyphenyl-substituent 9,9'-position (2,2-diphenylhydrazone) methyl substituent to 3,3'-bicarbazole derivatives The impact of the charge transfer characteristics. By introducing the electron acceptor and donor part into the 3,3'-bicarbazole structure material, the drift mobility of electrons and holes can reach about 1·10-5 cm2/V·s. Use the photoelectric effect experiment to determine the level of molecular ionization (If) and electron affinity (EAf) in the film. Depending on the nature of the substituents at the 6,6'- and 9,9'- positions, the If level ranges from -5.19 to -5.13 eV, and the EAf level ranges from -2.44 to -2.38 eV.A series of new copolymers of 3,3′-biscarbazolyl and pyridine have been synthesized using a convenient and straightforward polymerization method.
The polymers are thermally stable and soluble in a number of organic solvents which is important for their processability. The fluorescent spectra of polymers illustrate their blue light emitting properties. Electrochemical studies reveal that incorporation of 3,3′-bicarbazole and pyridine moieties can improve the hole and electron injection. Thermal stability, electrochemical and fluorescent properties signify the potential candidacy of these new copolymers as multifunctional materials for optoelectronic applications.
Synthesis of new copolymers of 3,3′-biscarbazolyl
Madiha Irfan et al.[5] research synthesis of the polymers ,which is presented graphically in figure 2.The N-alkylated bis-carbazolyl 1 was prepared by Nalkylation of the carbazole with octyl chain using the phase transfer catalyst in presence of base . The oxidative dimerization of N-octyl carbazole was achieved using anhydrous ferric chloride in dry chroroform . The biscarbazole 2 was subsequently subjected to acetylation using acetyl chloride and aluminum chloride in chloroform via the Fridel–Crafts acylation method providing the monomer 3 in 71% yield.
Polymers were synthesized by modified Chichibabin reaction by dissolving an equimolar mixture of monomer 3 and benzaldehydes in acetic acid (15 ml) and ammonium acetate (10 mmol) upon 30 h reflux . Fractionation was done by adding methanol, precipitates formed were filtered and dissolved in THF and re-precipitated by adding methanol, filtered through a short plug of basic alumina to remove traces of iron chloride and dried in vacuum oven overnight. The polymers obtained are soluble in most of the common organic solvents like CHCl3, CH2Cl2, THF as well as CH3CN and DMSO.
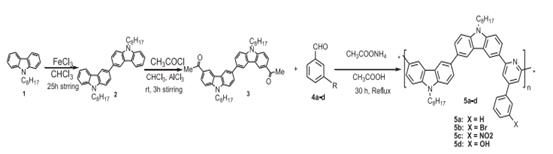

Figure 2 the synthesis of polymers
Reference
1.Saulius Grigalevicius. 3,6(2,7),9-Substituted carbazoles as electroactive amorphous materials for optoelectronics[J]. Synthetic Metals,2005,156(1).
2.Gang Liu, Der-Jang Liaw, Wei-Yi Lee et al. Tristable Electrical Conductivity Switching in a Polyfluorene-Diphenylpyridine Copolymer with Pendant Carbazole Groups[J]. Philosophical Transactions: Mathematical, Physical and Engineering Sciences,2009,367(1905).
3.Kim Jungwoon,Lee Suhan,Lee Jaemin,Lim Eunhee,Jung Byung Jun. 3,3'-Bicarbazole-Based Host Molecules for Solution-Processed Phosphorescent OLEDs.[J]. Molecules (Basel, Switzerland),2018,23(4).
4.Armands Ruduss, Kaspars Traskovskis, Elina Otikova,Aivars Vembris et al. 3,3'-Bicarbazole structural derivatives as charge transporting materials for use in OLED devices[P]. Photonics Europe,2018.
5.Madiha Irfan,Iqbal Ahmad,Shahid Ameen Samra,Aamer Saeed. Study on the synthesis and characterization of 3,3′-bicarbazole and pyridine based luminescent copolymers[J]. Materials Letters,2015,161:
See also
Lastest Price from 3,3'-Bicarbazole manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 1984-49-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton

US $0.00-0.00/kg2025-03-26
- CAS:
- 1984-49-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons