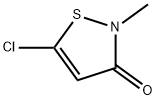
Application of 5-chloro-2-methyl-4-isothiazolin-3-one
5-chloro-2-methyl-4-isothiazolin-3-one is a 1,2-thiazole that is 4-isothiazolin-3-one bearing a methyl group on the nitrogen atom and a chlorine at C-5. It is a powerful biocide and preservative and is the major active ingredient in the commercial product Kathon(TM). It has a role as an antimicrobial agent, a xenobiotic and an environmental contaminant. It is a member of 1,2-thiazoles and an organochlorine compound. It derives from a methylisothiazolinone.
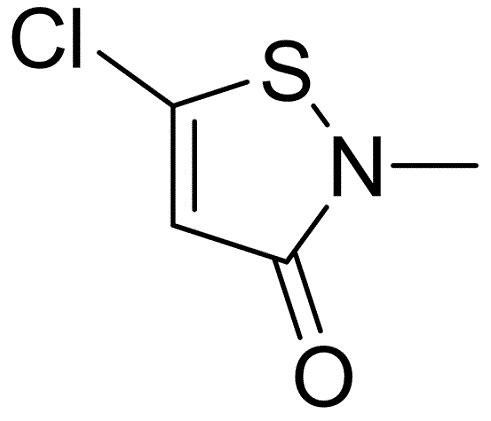

Application
5-chloro-2-methyl-4-isothiazolin-3-one (CMI) forms crystals. It is used as antimicrobial preservative in cosmetics, hygiene products, paints, emulsions, cutting oils, paper coatings, and water storage and cooling units. CMI is also used in hydraulic fracturing fluids:[1]
1.Methylchloroisothiazolinone (MCI) is an isothiazolinone commonly used as a preservative with antibacterial and antifungal properties. It is found within many commercially available cosmetics, lotions, and makeup removers. It is also a known dermatological sensitizer and allergen; some of its side effects include flaky or scaly skin, breakouts, redness or itchiness, and moderate to severe swelling in the eye area. The American Contact Dermatitis Society named Methylchloroisothiazolinone the Contact Allergen of the Year for 2013. Sensitivity to Methylchloroisothiazolinone may be identified with a clinical patch test.
2. Biocides are added to biodiesels to inhibit and remove microbial growth. The effects of 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT), a candidate biodiesel biocide, were studied using freshly isolated rat alveolar macrophages (AM) and NR8383 cell line. CMIT markedly inhibited phagocytic oxidative burst as measured by zymosan-induced chemiluminescence, and cellular cytokine secretion as measured by zymosan-induced TNF-α secretion.[2]
Toxicological and Safety
A risk assessment for safety management of CMIT/MIT was conducted on products containing 0.0015% of CMIT/MIT, which is the maximum MIT level allowed in current products. The no observed adverse effect level (NOAEL) for CMIT/MIT was 2.8 mg/kg bw/day obtained from a two-generation reproductive toxicity test, and the skin sensitization toxicity standard value for CMIT/MIT, or the no expected sensitization induction level (NESIL), was 1.25 µg/cm2/day in humans. According to a calculation of body exposure to cosmetics use, the systemic exposure dosage (SED) was calculated as 0.00423 mg/kg bw/day when leave-on and rinse-off products were considered. Additionally, the consumer exposure level (CEL) amounted to 0.77512 µg/cm2/day for all representative cosmetics and 0.00584 µg/cm2/day for rinse-off products only.
As a result, the non-cancer margin of safety (MOS) was calculated as 633, and CMIT/MIT was determined to be safe when all representative cosmetics were evaluated. In addition, the skin sensitization acceptable exposure level (AEL)/CEL was calculated as 0.00538 for all representative cosmetics and 2.14225 for rinse-off products; thus, CMIT/MIT was considered a skin sensitizer when all representative cosmetics were evaluated. Current regulations indicate that CMIT/MIT can only be used at concentrations 0.0015% or less and is prohibited from use in other cosmetics products. According to the results of this risk assessment, the CMIT/MIT regulatory values currently used in cosmetics are evaluated as appropriate.[3]
Biological and pharmacological activity
1.An isothiazolone biocide, 5-chloro-2-methyl-4-isothiazolin-3-one (CMI), was degraded in the presence of iron. According to the Fe-dependent degradation of CMI, stoichiometric production of chloride was observed. Copper and stainless steel did not enhance the physico-chemical degradation of CMI, whilst phosphate inhibited the Fe-dependent degradation. Neither aerobic nor anaerobic conditions influenced the Fe-dependent CMI degradation. Furthermore, FeO (OH)-powder and Fe3O4-powder did not lead to the physico-chemical degradation of CMI. Rapid disappearance of CMI was observed in an operating cooling water plant. Isothiazolone utilises a two-step mechanism in-volving rapid inhibition (within minutes) of growth and metabolism, followed by irreversible cell damage resulting in loss of viability. Isothiazolones are stable under typical environmental conditions, but are degraded by strong nucleophiles, such as sulfide or alkanolamines
2. Methylchloroisothiazolinone is approved for use within allergenic epicutaneous patch tests which are indicated for use as an aid in the diagnosis of allergic contact dermatitis (ACD) in persons 6 years of age and older.
Reference
[1]
[2]Tanji Y., Nishihara T. & Miyanaga K., "Iron dependent degradation of an isothiazolone biocide (5-chloro-2-methyl-4-isothiazolin-3-one)," Biofouling (Chur, Switzerland), Vol.23, No.2(2007), pp.73-77.
[3]
You may like
Related articles And Qustion
See also
Lastest Price from 5-Chloro-2-methyl-4-isothiazolin-3-one manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 26172-55-4
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 10MT

US $1.00/KG2025-04-21
- CAS:
- 26172-55-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt