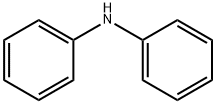
The biodegradability of diphenylamine
Introduction
Diphenylamine (DPA) is a compound from the third European Union (EU) list of priority pollutants. It was as[1]signed by the EU to Germany to assess and control its environmental risks. DPA and derivatives are most commonly used as stabilizers in nitrocellulose-containing explosives and propellants, in the perfumery, and as antioxidants in the rubber and elastomer industry. DPA is also widely used to prevent post-harvest deterioration of apple and pear crops. DPA is a parent compound of many derivatives, which are used for the production of dyes, pharmaceuticals, pho[1]tography chemicals and further small-scale applications. Diphenylamines are still produced worldwide by the chemical industries. First reports showed that DPA was found in soil and groundwater. Some ecotoxicological studies dem[1]onstrated the potential hazard of various diphenylamines to the aquatic environment and to bacteria and animals[1].

Picture 1 Diphenylamine powders
The biodegradability of DPA
Studies on the biodegradability of DPA and its derivatives are very sparse. Therefore, further investigation is required to determine the complete dimension of the potential environmental hazard and to introduce possible (bio)remediation techniques for sites that are contaminated with this class of compounds. This is the first detailed review on DPA and some derivatives summarizing their environmental relevance as it is published in the literature so far and this review will recommend conducting further research in the future.
Application
The worldwide annual production of DPA in the 1980s was about 40 000 t of which nearly 4000 t were produced in Germany[1]. The compound still has an industrial significance, so that the todays annual production amount may be even higher. This is especially supported by published values showing high production rates for this compound in the eastern countries of Europe (for example, the Slovak Republic producing more than 10 000 t/year[2]. [1]
DPA is predominantly used as stabilizer for single- or multi-base propellants and nitrocellulose-containing explosives. It has the function of binding degradation products, which develop during long-term storage (e.g., NO, NO2, HNO3) in order to prolong storage times. Otherwise, the nitric degradation products would en[1]hance further decomposition so that finally the powder becomes useless [3]. During its stabilizing reaction, DPA is transformed to its nitrated derivatives (above all to mono-, di- and trinitro-DPA) [4]. If DPA from ammunition waste causes soil and water contamination, it always will be accom[1]panied by nitrated DPA derivatives. For example, at a site of a former ammunition plant in Lower Saxony (Germany), in which nitrocellulose had been produced and mixed with DPA as stabilizer during the second world war, Haas et al. (1990) could detect large fractions of contaminated material.
Gunpowder plates or discs were found at this site, which showed high concentra[1]tions of DPA (1.4–2.9 g/kg), 2-n g/kg), and 4-nitro-DPA (0.4–1.2 g/kg). In another study, Entenmann and Sch€acke (1994) reported on a former military air base area in Lower Saxony (Germany) on which a widely scattered, diffuse contamination with DPA, 2-nitro-DPA, and 4-nitro-DPA in soil and in groundwater had been detected. In general, the detection of DPA and derivatives has not found analytical consideration in most cases until now, although the analytical techniques are established (see references below). DPA is also widely used to prevent post-harvest de[1]terioration (storage scald) of apple and pear crops [5]. Another important application of DPA is its use as an antioxidant for various polymers and elastomers and as condensates for the insulation of rubber. Some further applications include: (i) DPA as precursor for the chemical synthesis of (azo-)dyes such as Metanil Yellow and Orange IV [6], (ii) DPA as stabilizer in perfumery products[7], (iii) its use for the detection of oxidizers (Sugihara et al., 1993), (iv) its use for the detection of DNA[8], (v) its use as a biozid against body louse, chiggers and housefly [9].
Because of the manifold utilization of DPA and derivatives there is a lot of literature available for the analytical detection of these compounds. Spectrophotometric, voltametric and amperometric, colorimetric as well as numerous chromatographic methods have been applied (TLC, HPLC, GC, and GC/MS) [10].
DPA is also described as a naturally occurring compound in onions[11], in leaves of black and green tea and further plants [12] as well as in the peel of citrus fruits. Therefore, the development of microbial degradation strategies for this compound could have occurred during microbial evolution[12] .
Reference
1 Ardito, G., Bramanti, B., Bigatti, P., Lamberti, L., Dolara, P., 1996. Cytogenetic effect thiabendazole and diphenylamine on cultured human lymphocytes: sister chromatid exchanges and cell cycle delay. Boll. Soc. Ital. Biol. Sper. 72, 171–178.
2 Bazin, B.H., Foussereau, J., Cavelier, C., 1986. Allergy to diphenylamine from an industrial grease. Contact Derma[1]titis 14, 116.
3 Bergens, A., 1995. Application of a numerical[1]model to concentration-time data for diphenylamine and its primary degradation products determined by liquid-chro[1]matography and dual-wavelength detection. Talanta 42, 185–196.
4 Bergens, A., Danielsson, R., 1995. Decomposition of diphenyl[1]amine in nitrocellulose based propellants. 1. Optimization of a numerical-model to concentration-time data for diphenyl[1]amine and its primary degradation products determined by liquid-chromatography with dual-amperometric detection. Talanta 42, 171–183.
5 Blotevogel, K.-H., Gorontzy, T., 2000. Microbial degradation of compounds with nitro functions. In: Rehm, H.-J., Reed, G. (Eds.), Biotechnology, second ed., vol. 11b. Wiley–VCH, Weinheim, Germany, pp. 273–301.
6 BUA (Beratergremium fur umweltrelevante Altstoffe der Ge- € sellschaft Deutscher Chemiker), 1993. BUA-Stoffbericht 114 (Erg€anzungsberichte I.), Diphenylamin (Nr. 15). S. Hirzel Verlag, Stuttgart, Germany. Burkinshaw, S.M., Lu, J.G., 1993.
7 Dyeing in the presence of free radical initiators. Part 3: The dyeing of Nylon 6.6 with nitrodiphenylamine and azo disperse dyes. Dyes Pigments 22, 131–149.
8 Calnan, C.D., 1978. Diphenylamine. Contact Dermatitis 4, 301. Christodoulatos, C., Koutsospyros, A., Brodman, B.W., Kor[1]fiatis, G.P., 1997. Biodegradation of diphenylamine by se[1]lected microbial cultures. J. Environ. Sci. Health A 32, 15–30.
9 Crocker, J.F.S., Brown, D.M., Borch, R.F., Vernier, R.L., 1972. Renal cystic disease induced in newborn rats by diphenylamine derivatives. Am. J. Pathol. 66, 343–350.
10 Curtis, N.J., Rogasch, P.E., 1987. Determination of derivatives of diphenylamine in Australian gun propellants by high performance liquid chromatography. Propell. Explos. Pyrot. 12, 158–163.
11 Das, B.R., Deardorff, M.B., Roberts, W.C., 1992. Health advisory for diphenylamine (DPA). US Environmental Protection Agency, Washington, DC, USA.
12 Decallonne, J.R., Weyns, J.C., 1976. A shortened procedure of the diphenylamine reaction for the measurement of deoxy[1]ribonucleic acid by using light activation. Anal. Biochem. 74, 448–456.
You may like
See also
Lastest Price from Diphenylamine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 122-39-4
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $24.00-296.00/kg2025-02-08
- CAS:
- 122-39-4
- Min. Order:
- 1kg
- Purity:
- 0.98
- Supply Ability:
- 100kg