Application and Pharmacology of Minoxidil
General description
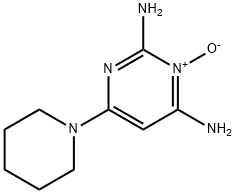
Minoxidil is an orally administered vasodilator with hair growth stimulatory and antihypertensive effects. Minoxidil is converted into its active metabolite minoxidil sulphate by sulphotransferase enzymes. Minoxidil sulphate exerts its antihypertensive effect by opening of plasma membrane adenosine triphosphate (ATP)-sensitive potassium channels (KATP channels), thereby directly and rapidly relaxing arteriolar smooth muscle and subsequent reduction of elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance. This agent's hair growth stimulatory effect may be mediated through its vasodilatory activity, thereby increasing cutaneous blood flow, or due to its direct stimulatory effect on hair follicle cells and forcing them from their resting phase into their active growth phase. Minoxidil is an antihypertensive agent that is used largely for patients with severe and refractory hypertension not responding to conventional therapies. Minoxidil is also used topically to treat male pattern baldness. Despite its use for many years, minoxidil has not been convincingly linked to cases of clinically apparent liver injury. Minoxidil is a pyrimidine N-oxide that is pyrimidine-2,4-diamine 3-oxide substituted by a piperidin-1-yl group at position 6. It has a role as a vasodilator agent and an antihypertensive agent. It is a pyrimidine N-oxide, a member of piperidines and an aminopyrimidine.
History
Minoxidil was first introduced as an antihypertensive medication and the discovery of its common adverse event, hypertrichosis, led to the development of a topical formulation for promoting hair growth. To date, topical minoxidil is the mainstay treatment for androgenetic alopecia and is used as an off-label treatment for other hair loss conditions. Despite its widespread application, the exact mechanism of action of minoxidil is still not fully understood. Minoxidil was first introduced as an oral medication for the treatment of severe and recalcitrant hypertension in the 1970s.1 Coincidentally, physicians observed hair regrowth and generalized hypertrichosis in balding patients, which led to the development of a topical minoxidil formulation for treating androgenetic alopecia (AGA) first in male and then in female individuals. The 2% minoxidil solution was first launched in the market in 1986, followed by the 5% solution in 1993.2 Despite its global acceptance for over 30 years, the mechanism underlying the hair growth-promoting effects of minoxidil remains to be fully elucidated.
Application and Pharmacology
Minoxidil is a piperidino-pyrimidine derivative, with the following chemical structure: 2,6-diamino-4-piperidinopyrimidine-1-oxide (C9H15N5O).2 Minoxidil solution (MS) contains inactive ingredients, including water, as well as ethanol and propylene glycol (PG), which are used as vehicles to enhance the solubility of minoxidil.3 PG facilitates efficient drug delivery into the hair follicles; however, its frequent induction of local irritation led to the development of a PG-free minoxidil foam (MF). The non-medical ingredients in the foam formulation include cetyl alcohol, stearyl alcohol, and butylated hydroxytoluene. Minoxidil in the treatment of androgenetic alopecia. The National Institutes of Health (US NIH, 2018) estimates that in the US approximately 50 million men and 30 million women suffer from AGA (also known as pattern hair loss). Minoxidil is the only topical drug for the treatment of both female and male pattern hair loss. In the US, minoxidil is approved over-the-counter (OTC) at a maximum concentration of 5%[1].
Synthesis
4-chloro-2,6-diaminopyrimidine was obtained by cyclization and chlorination of methyl cyanoacetate, and then oxidized with perbenzoic acid to obtain 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-chloropyrimidine (c4h5cin4o). Finally, minoxidil was prepared by condensation with Hexahydropyridine.
Safety
Minoxidil is a common medication prescribed for treating hair loss-related problems. It provides remarkable benefits to patients with hair disorders. To date, the FDA has approved minoxidil only for AGA. However, minoxidil is used off-label for treating several hair disorders as well as increasing body hair growth. Although topical minoxidil is considered an effective and safe treatment option for various hair disorders, additional evidence-based data are needed for some applications[2].
Reference
1.Goren A. & Naccarato T., "Minoxidil in the treatment of androgenetic alopecia," Dermatologic Therapy, Vol.31, No.5(2018), p.e12686.
2.Suchonwanit P., Thammarucha S. & Leerunyakul K., Minoxidil and its use in hair disorders: a review, Vol.Volume 13(2019), pp.2777-2786.
You may like
Related articles And Qustion
See also
Lastest Price from Minoxidil manufacturers

US $0.00-0.00/kg2025-10-31
- CAS:
- 38304-91-5
- Min. Order:
- 1kg
- Purity:
- USP/EP/BP
- Supply Ability:
- 20TONS
US $0.00/kg2025-10-12
- CAS:
- 38304-91-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS