The use of minoxidil in hair disorders
Introduction
Minoxidil is a common medication prescribed for treating hair loss-related problems. It provides remarkable benefits to patients with hair disorders. To date, the FDA has approved minoxidil only for AGA. However, minoxidil is used off-label to treat several hair disorders and increase body hair growth. Minoxidil was first introduced as an oral medication for the treatment of severe and recalcitrant hypertension in the 1970s. Coincidentally, physicians observed hair regrowth and generalized hypertrichosis in balding patients, which led to the development of a topical minoxidil formulation for treating androgenetic alopecia (AGA) first in male and then in female individuals. The 2% minoxidil solution was first launched in 1986, followed by the 5% solution in 1993[1].
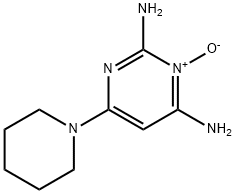
it is a piperidine-pyrimidine derivative with the following chemical structure: 2,6-diamino-4-piperidinopyrimidine-1-oxide (C9H15N5O). Minoxidil solution (MS) contains inactive ingredients, including water, ethanol, and propylene glycol (PG), which are used as vehicles to enhance the solubility of minoxidil. PG facilitates efficient drug delivery into the hair follicles; however, its frequent induction of local irritation led to developing a PG-free minoxidil foam (MF). The non-medical ingredients in the foam formulation include cetyl alcohol, stearyl alcohol, and butylated hydroxytoluene[2].
Use

Androgenetic alopecia (AGA)
AGA is a non-scarring alopecia in which terminal hairs transform into miniaturized hairs. Typically, in men, baldness occurs with frontal recession and vertex thinning. At the same time, in women, hair loss is characterized by a decrease in hair density over the crown without frontal hairline involvement, also known as female pattern hair loss (FPHL). Testosterone plays a role in AGA pathogenesis following conversion to dihydrotestosterone (DHT) by 5-alpha reductase.
A meta-analysis showed that topical minoxidil at all concentrations provided superior results to those in the placebo group. Regarding AGA, the study showed mean differences of 8.11 hairs/cm2 and 14.90 hairs/cm2 associated with the 2% and 5% minoxidil treatments, respectively, compared to the placebo group. A comparison of the 2% minoxidil and placebo groups in female patients showed a mean difference of 12.41 hairs/cm2. A 5-year follow-up study in AGA patients treated with 2% and 5% MS showed peak hair regrowth at 1 year.
Scarring alopecia
In scarring alopecia, medical treatment should be initiated as early as possible. The treatment aims to preserve the remaining hair follicles and halt disease progression. Central centrifugal cicatricial alopecia is a common scarring alopecia among African American women. It usually presents with hair loss on the vertex and spreads toward the periphery. In one small retrospective study, a combination of high-potency topical steroids and topical minoxidil showed no significant improvement. However, a decrease in disease severity scores in some patients might suggest that the medication can slow the course of the condition. Topical minoxidil has been used to treat frontal fibrosing alopecia, a scaring alopecia condition affecting the frontal and temporal hairlines.
Alopecia areata
AA is an autoimmune disease of the hair follicles with a clinical presentation ranging from patchy, non-scarring alopecia to complete scalp (alopecia totalis) and body (alopecia universalis) hair loss. Many treatment modalities are available; nonetheless, none has received FDA approval. Minoxidil is occasionally used off-label as monotherapy or in combination with other treatments. Many studies have suggested that topical minoxidil offered some benefits to AA patients as it slightly increased hair growth without altering disease progression or inducing remission. As a monotherapy, topical minoxidil treatment failed to demonstrate a statistically significant improvement. Thus, it has been recommended as an adjuvant therapy for AA.
Hair shaft disorders
Minoxidil has been used to treat monilethrix, a rare autosomal dominant hair disorder manifested as fragile hair shafts with a regular beaded appearance. Oral minoxidil has been used in many cases of hair shaft disorders, as topical minoxidil application may worsen hair shaft brittleness.
Chronic telogen effluvium
Telogen effluvium is a common non-scarring alopecia characterized by excessive telogen hair shedding triggered by stressful events such as pregnancy, a significant illness, and surgery. Chronic telogen effluvium (CTE) is hair loss persisting over 6 months. Treatment for CTE can be frustrating, and many medications have been tried, including minoxidil. There have been limited clinical trials on topical minoxidil use to treat telogen effluvium. Oral minoxidil may be a promising treatment option. However, Some patients demonstrated mild facial hypertrichosis, dizziness, and altered blood pressure as adverse events.
References
[1] P. Suchonwanit, Kanchana Leerunyakul, Sasima Thammarucha. “Minoxidil and its use in hair disorders: a review.” Drug Design, Development and Therapy 13 1 (2019): 2777–2786.
[2] MBBS, Jared M. John et al. “Systemic minoxidil for hair disorders in pediatric patients: a safety and tolerability review.” International Journal of Dermatology 62 2 (2022): 257–259.
You may like
Related articles And Qustion
See also
Lastest Price from Minoxidil manufacturers

US $0.00-0.00/kg2025-10-31
- CAS:
- 38304-91-5
- Min. Order:
- 1kg
- Purity:
- USP/EP/BP
- Supply Ability:
- 20TONS
US $0.00/kg2025-10-12
- CAS:
- 38304-91-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS