Application and Pharmacology of Eugenol
General description
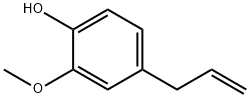
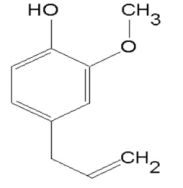
Eugenol is a phenylpropane compound existing in Syringa plants. It is a liquid with lilac flower flavor. Its chemical name is 4-allyl-2-methoxyphenol. Eugenol is the main bioactive compound of Syringa (Myrtaceae). Fresh Syringa contains about 9.3%~14.7% eugenol.
Eugenol, also called clove oil, is an aromatic oil extracted from cloves that is used widely as a flavoring for foods and teas and as an herbal oil used topically to treat toothache and more rarely to be taken orally to treat gastrointestinal and respiratory complaints. Eugenol in therapeutic doses has not been implicated in causing serum enzyme elevations or clinically apparent liver injury, but ingestions of high doses, as with an overdose, can cause severe liver injury. Eugenol is a phenylpropanoid formally derived from guaiacol with an allyl chain substituted para to the hydroxy group. It is a major component of clove essential oil, and exhibits antibacterial, analgesic and antioxidant properties. It has been widely used in dentistry to treat toothache and pulpitis. It has a role as an allergen, a human blood serum metabolite, a sensitiser, a volatile oil component, a flavouring agent, an EC 1.4.3.4 (monoamine oxidase) inhibitor, a radical scavenger, an antibacterial agent, an antineoplastic agent, an apoptosis inducer, an anaesthetic, an analgesic, a voltage-gated sodium channel blocker, a NF-kappa B inhibitor and an anti-inflammatory agent. It is a phenylpropanoid, a monomethoxybenzene and a member of phenols. It derives from a guaiacol. Eugenol appears as clear colorless pale yellow or amber-colored liquid. Odor of cloves. Spicy pungent taste. Harbin City clove is the flower of Syringa in Oleaceae. The volatile compounds of Syringa plants mainly contain eugenol, syringol isomer and syringaldehyde isomer as the characteristic components of Spicy aroma. The smell of eugenol is pungent, like Syringa aroma, smoke aroma and sweet aroma[1].
Application and Pharmacology
1.Eugenol is a bioactive compound widely available in many herbs like clove, cinnamon, tulsi, pepper etc. The compound is known for its antioxidant, antimicrobial, anesthetic, anti-inflammatory, neuroprotective,anti-diabetic,andanti-canceractivities.Inpharmaceuticalanalysis, eugenol is used as a marker for single drugs and drug products. Dental care, household, and personal hygiene products are other areas where it has established its potential. In the food industry, eugenol is used as a flavouring agent in non-alcoholic beverages, baked foods, and chewing gums[2].
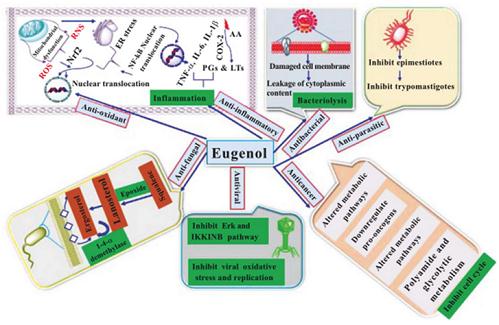
Figure 1 Pharmacological action of eugenol as anti-oxidant, antibacterial, antifungal, anti-viral, anti-inflammatory, anti-parasitic and anti-cancer agent
2.Eucalyptol, also known as 1,8-cineole, is a terpenoid oxide isolated from Eucalyptus species such as Eucalyptus globules Labill. and Eucalyptus tereticornis Sm. Eucalyptol is derived from the leaf oil of these plants, which contains various volatile organic components. Terpenes such as eucalyptol are lipophilic molecules that disturb intracellular lipids and increase drug penetration. A lipid-based microemulsion system of eucalyptol has been utilized for transdermal drug delivery. Eucalyptol is metabolized to 2-exo-hydroxy-1,8-cineole by rat and human liver microsomal P450 enzymes and eliminated in the urine. Moreover, eucalyptol can easily pass through the blood–brain barrier and may have direct action on receptors and enzymes in the brain.Eucalyptol has been reported to have antimicrobial, anti-inflammatory, antioxidant, analgesic, and spasmolytic effects in various diseases including colds, influenza, other respiratory infections, rhinitis, and sinusitis. Eucalyptol acted as a strong inhibitor of proinflammatory cytokines such as tumor necrosis factor (TNF)-a and interleukin (IL)-1b and showed an analgesic effect in an inflammatory model. Eucalyptol significantly increased the beat frequency of nasal cilia in mucus membranes and had bronchodilation effects. In addition, it decreased exacerbation in asthma, sinusitis, and COPD symptoms by inhibiting cytokine-induced airway mucus hypersecretion. It exhibits antioxidant activity by radical scavenging and reduces Ca2+ influx via calcium channels in cardiac muscle.
3.Eucalyptol was shown to have effects in various inflammatory diseases including espiratory diseases, pancreatitis, and cardiovascular and neurodegenerative disease as well as reducing colon damage. In particular, it is reported that eucalyptol has been studied in animal and human model-related chronic disease. Eucalyptol is used in inflammatory airway diseases as a mucolytic agent. Eucalyptol treatment significantly reduced dyspnea and enhanced lung function and quality of life relative to placebo in patients with stable COPD. Eucalyptol treatment also resulted in improvements in patients with asthma, a disease characterized by a chronic inflammatory process, by enhancing lung function and general health. Moreover, eucalyptol has been used to treat chronic bronchitis, sinusitis, and rhinitis.Eucalyptol exerts anti-inflammatory and antioxidative effects by regulating the NF-jB and MAPK signaling pathways in several diseases, including chronic diseases. These beneficial effects of eucalyptol have been observed in clinical studies and in several animal models. Eucalyptol, which has lipophilic properties and exerts various actions on receptors and enzymes, may be a potentially important drug in the treatment of chronic diseases.
Synthesis
1.The interaction of organoacetoxysilanes with menthol, eugenol, vanillin, citronellol, phenol, cyclohexanol and hexanol was investigated. Products of full and partial esterification were obtained. The hydrolysis of alkoxy(aroxy)silanes and acetoxyalkoxy(aroxy)silanes in a solution of Me Et ketone or THF and on a cellulose surface was investigated. Rates of acetoxyalkoxy(aroxy)silane hydrolysis on the cellulose surface were by 1-2 orders lower than in a solution, but the dependence on the nature of the substituents remained.
2.The invention relates to the preparation of vanillin from eugenol, which has the following advantages: mild reaction conditions, high conversion rate, high selectivity, high yield, no waste water, and environmental protection. For example, vanillin was prepared by the following steps: isomerization of eugenol; esterification with acetic anhydride; oxidation and reduction; alcoholysis to obtain vanillin.
Safety
At first, eugenol was used as a perfume. Later, it was found to have insect repellent and antibacterial effects, as well as its good anti-tumor activity, indicating that eugenol has a great development prospect. In terms of pharmacological activity, anti-tumor effect of eugenol can not be ignored. MF et al. found that eugenol has anti proliferation effect on human cervical cancer cells, which is mainly achieved by inducing apoptosis. ISS et al. [6] found that eugenol was added to the anticancer drug cisplatin. They found that eugenol could enhance the inhibitory effect of cisplatin on breast cancer stem cells by inhibiting ALDH enzyme activity. At the same time, the study showed that eugenol enhanced the effect of cisplatin on NF- κ Inhibition of B signal pathway.
Reference
1. Taleuzzaman, M., et al., Eugenol as a Potential Drug Candidate: A Review. Current Topics in Medicinal Chemistry, 2021. 21(20): p. 1804-1815.
2. Fujisawa, S. and Y. Murakami, Eugenol and Its Role in Chronic Diseases. Adv Exp Med Biol, 2016. 929: p. 45-66.
You may like
Related articles And Qustion
See also
Lastest Price from Eugenol manufacturers

US $2.00/L2025-09-28
- CAS:
- 97-53-0
- Min. Order:
- 2L
- Purity:
- 99%
- Supply Ability:
- 20tons per month

US $3.00/kg2025-04-21
- CAS:
- 97-53-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000