Application and environmental risk assessment of climbazole
General description
Gamboline is white or off white crystalline or crystalline powder. It is easy to dissolve in toluene and alcohol, but difficult to dissolve in water. Gambolin has broad-spectrum bactericidal properties[1]. It is mainly used in antipruritic and dandruff conditioning shampoo and hair care shampoo. It can also be used in antibacterial soap, shower gel, medicated toothpaste, mouthwash and other high-end washing products[2].
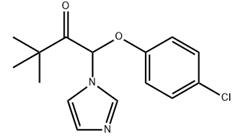
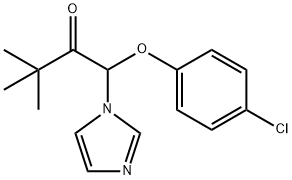
Fig. 1 The structure of climbazole
The detailed structure of climbazole
New multicomponent crystals of climbazole (CLB) with fumaric acid (FumAc) and tioconazole (TCL) with oxalic (OxlAc), malonic (MlnAc), fumaric, and DL-tartaric (TartAc) acids were obtained by liq.-assisted grinding. The single crystals of CLB were obtained for the first time, and the crystal structure was characterized. The crystal structures of the four multicomponent crystals studied-[CLB + FumAc] cocrystal (2:1), [TCL + MlnAc] salt (1:1), [TCL + FumAc] salt (1:1), and [TCL + TartAc + H2O] salt (1:1:1)-the single crystals of which were obtained by slow evapn. form soln. were detd. The influence of the coformer structure of the multicomponent crystals on CLB and TCL conformational flexibility was discussed. A thermal anal. of the studied multicomponent crystals was carried out. The [TCL + TartAc + H2O] salt (1:1:1) dehydration mechanism was analyzed by differential scanning calorimetry, a thermogravimetric method, and thermomicroscopy[3-4]. It was established that the completion of the [TCL + TartAc + H2O] salt (1:1:1) dehydration process was followed by melting of the eutectic mixt. and crystn. of the [TCL + TartAc] salt (1:1). A study of the multicomponent crystal soly. was carried out in a phosphate buffer soln. at pH 6.8. Cocrystn./salt formation of the studied active pharmaceutical ingredients with dicarboxylic acids not only significantly improved their soly. but also maintained the level of supersatn. in the soln. for quite a long time[5].
Mechanism of potential toxicity of climbazole to male zebrafish
As the main active ingredient for the treatment of fungal infections, climbazole (CBZ) is commonly used in a variety of personal care products. After its use, CBZ enters the receiving environment directly or indirectly through domestic sewage. Its concn. can be up to several nanograms per L in surface water[6]. So far, the effects of CBZ on the reproductive system of female zebrafish have been systematically studied, but the potential toxicity mechanism of CBZ on male zebrafish still needs to be further explored. In this study, adult male zebrafish were exposed to CBZ at concns. of 0.1, 10, and 1000μg·L-1 for 28 days, and their testes were collected for histol., mass-spectrometry-based metabolomics, and biochem. analyses. We found that CBZ caused a significantly abnormal metab. of purine and glutathione and triggered oxidative stress in zebrafish testes, thereby inducing testicular cell apoptosis[7]. In addition, CBZ could inhibit the synthesis of essential sex hormones in the testis and thus reduce the sperm prodn. The conclusions of this study fill the data gap on the reproductive toxicity of CBZ to male zebrafish and highlight the ecotoxicol. application of untargeted metabolomics in the biomarker discovery[8].
Risks to the environment
Climbazole (CBZ) is an emerging recalcitrant contaminant in wastewater, which has severe toxic effects on aquatic organisms. In this study, the monodispersed Fe3-xCuxO4 nanoparticles were fabricated and used as the heterogeneous catalyst for activating peroxymonosulfate (PMS) toward the degrdn. of CBZ in aq. Solns[9-10]. The results revealed that Cu incorporation in magnetite led to a larger surface area, more reductive ions, and oxygen vacancies on the surface, which effectively improved the catalytic efficiency. For a wide range of pH values (2.2-11.0), the optimized Fe2.31Cu0.69O4 achieved the most excellent efficiency and stability in terms of activating PMS toward CBZ degrdn. Based on the detections of ESR spectroscopy (ESR) and radical scavenger tests, sulfate radicals (SO4·-) were identified as the main active species during the CBZ degrdn. process. The possible mechanisms whereby Cu enhances the electron transfer and the generation of superoxide radicals (O2·-) during the activation process of PMS by Fe3-xCuxO4 were proposed[11]. Ten degraded products produced from CBZ through the hydroxylation, dechlorination, ether chain cleavage, and dealkylation pathways were identified. Most of the byproducts' acute and chronic toxicities were reduced to a much lower level than that of CBZ. The obtained results provide an avenue for rationally constructing and developing a catalyst for the efficient treatment of azole fungicides in wastewater[12].
Action mechanism of climbazole on environment
Widespread use of azole fungicides and low removal efficiency in wastewater treatment plants (WWTPs) have led to the elevated concn. of azole fungicides in receiving environment. However, there was limited research about the removal mechanism of azole fungicides in the biol. treatment of WWTPs[13]. Imidazole fungicide climbazole and triazole fungicide fluconazole were selected to investigate the biodegrdn. mechanism of azole fungicides in activated sludge under aerobic conditions. I was found to be adsorbed to solid sludge and resulted in quick biodegrdn. The degrdn. of climbazole in the aerobic activated sludge system was fitted well by the first-order kinetic model with a half-life of 5.3 days, while fluconazole tended to stay in liq. and had only about 30% of loss within 77 days incubation. Ten biotransformation products of climbazole were identified by high resoln. mass spectrometry using suspect and non-target screening method[14-15]. But no biodegrdn. products of fluconazole were identified due to its limited removal. The possible biodegrdn. pathways for climbazole were proposed based on the products identification and pathway prediction system, and involves oxidative dehalogenation, side chain oxidn. and azole ring loss. The findings from this study suggest that it should be a concern for the persistence of fluconazole in the environment[16].
Reference
[1] S. Blokhina, A. Sharapova, M. Ol'khovich, G. Perlovich, Experimental and thermodynamic study of solubility, partition and solvation of climbazole, J. Therm. Anal. Calorim. (2022) Ahead of Print.
[2] L.H.D. Do, R.M. Law, H.I. Maibach, Dose response effect of chemical surface concentration on percutaneous penetration in human: In vivo + in vitro, Regul. Toxicol. Pharmacol. 132 (2022) 105186.
[3] C. Koch, S. Lange, N. Bugdahn, Cosmetic compositions comprising antimicrobials and (bio)-alkanediols for skin protection, Symrise AG, Germany . 2022, p. 264pp.
[4] C. Koch, S. Lange, N. Bugdahn, Cosmetic compositions comprising antimicrobials and (bio)-alkanediols for skin protection, Symrise AG, Germany . 2022, p. 203pp.
[5] C. Koch, S. Lange, N. Bugdahn, R. Kraeling, M. Mascher, Cosmetic compositions comprising natural polymers and (bio)-alkanediols, Symrise AG, Germany . 2022, p. 193pp.
[6] C. Koch, S. Lange, R. Kraeling, M. Mascher, N. Bugdahn, Cosmetic compositions comprising natural polymers and one or more (bio)-alkanediols, Symrise AG, Germany . 2022, p. 247pp.
[7] S. Lange, N. Bugdahn, C. Koch, Cosmetic compositions comprising (bio)-alkanediols with antimicrobials for product protection, Symrise AG, Germany . 2022, p. 282pp.
[8] S. Lange, N. Bugdahn, C. Koch, Cosmetic compositions comprising (bio)alkanediols with antimicrobials for product protection, Symrise AG, Germany . 2022, p. 204pp.
[9] S. Lange, N. Bugdahn, R. Kraeling, Cosmetic compositions comprising one or more (bio)-alkanediols with active ingredients, Symrise AG, Germany . 2022, p. 281pp.
[10] S. Lange, N. Bugdahn, R. Kraeling, Cosmetic compositions comprising one or more (bio)-alkanediols with active ingredients, Symrise AG, Germany . 2022, p. 218pp.
[11] J. Liu, Y. Pi, Hair care compositions comprising an anti-dandruff agent and a polymer, Unilever IP Holdings B.V., Neth.; Unilever Global IP Limited; Unilever PLC . 2022, p. 25pp.
[12] W. Liu, Dandruff removing composition and shampoo containing the dandruff removing composition and its preparation method, Guangzhou Jinxiang Cosmetics Co., Ltd., Peop. Rep. China . 2022, p. 20pp.
[13] A. Piacentini, M. Koehler, V. Kallmayer, M. Bernau, A. Ponnudurai, New cosmetic solvents comprising ascorbic acid, Beiersdorf A.-G., Germany . 2022, p. 12pp.
[14] M.A. Plescia, R.A. Ganz, M.E. Saremi, Deodorant compositions, G&S Laboratories, Inc., USA . 2022, pp. 39pp., Cont. of U.S. Provisional Ser. No. 126,498.
[15] Y. Wang, N. Wen, Based on cambium comprehensive catalytic device and catalytic method [Machine Translation], Chizhou Wanwei Chemical Co., Ltd., Peop. Rep. China . 2022, p. 13pp.
[16] W. Xiao, M. Pahlavanneshan, C.-Y. Eun, X. Zhang, C. DeKalb, B. Mahgoub, H. Knaneh-Monem, S. Shah, A. Sohrabi, S.K. Seidlits, R. Hill, Matrix stiffness mediates pancreatic cancer chemoresistance through induction of exosome hypersecretion in a cancer associated fibroblasts-tumor organoid biomimetic model, Matrix Biol. Plus 14 (2022) 100111.
Related articles And Qustion
See also
Lastest Price from Climbazole manufacturers
US $0.00/kg2025-11-21
- CAS:
- 38083-17-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/kg2025-08-26
- CAS:
- 38083-17-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons