Androstenedione: Occurrence and Metabolism
General Description
Androstenedione, a naturally occurring steroidal hormone, is produced by the gonads and adrenal glands in both males and females. It serves as an intermediate in testosterone biosynthesis and plays a vital role in bone health. Research has explored androstenedione metabolism in fungi, particularly Absidia coerulea, revealing insights into steroid biotransformation pathways. In humans and non-human primates, androstenedione undergoes complex metabolism involving various tissues and enzymatic reactions, contributing to the synthesis of testosterone and estrone. Understanding its metabolism is crucial for personalized medicine and managing hormone-related diseases, given its potential impact on endogenous hormone levels.

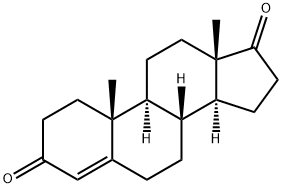
Figure 1. Androstenedione
Occurrence
Androstenedione, also known as 4-Androstene-3-17-dione (4A), is a naturally occurring steroidal hormone found in both males and females, produced by the gonads and adrenal glands. It serves as an intermediate in the biosynthesis of testosterone, a key hormone involved in various physiological functions. The production of androstenedione primarily occurs under normal conditions in the adrenals (2-3 mg/day) and testes (0.5 mg/day). Androgens, including androstenedione, play a crucial role in bone health, influencing both bone maturation and the maintenance of mature bones. Deficiency in androgens has been linked to premature bone loss and an increased prevalence of osteoporosis in men. Treatment with anabolic steroids has been shown to positively impact bone mass in postmenopausal women. In vitro studies have demonstrated that androgens stimulate the proliferation of human and murine osteoblast-like cells, indicating a direct effect on bone cells through receptor-mediated mechanisms. This highlights the importance of androstenedione in bone health and the overall physiology of mammals. 1
Metabolism
Metabolism in Fungi
In a study led by researchers, the metabolism of androstenedione in fungi, particularly in the cultured strain of Absidia coerulea, was investigated. This research focused on the biotransformation of testosterone, androstenedione, and progesterone derivatives within the fungi strain. The substrates underwent transformation and hydroxylation processes, leading to the isolation of various biotransformed metabolites. Additionally, the study extended to include derivatives of these steroids with specific structural features such as an extra C1-C2 double bond and/or a 17 α-methyl group. By utilizing Absidia coerulea as a model organism, the researchers aimed to explore the eukaryotic biotransformation of these steroids. Despite similarities in the 4-ene-3-oxo system among the substrates, differences arose from variations in substituents at C-17 and the presence of an additional C1-C2 double bond. This comprehensive investigation sheds light on the intricate metabolic pathways of androstenedione within fungi, contributing valuable insights to steroid biotransformation studies. 2
Metabolism in Humans and Non-Human Primate
Androstenedione, a key intermediate in steroid hormone biosynthesis, undergoes complex metabolism in humans and non-human primates. It circulates in the body along with other 11-oxygenated steroids, with higher levels detected in humans and non-human primates compared to other species. The synthesis of androstenedione and its derivative, androstadienedione, plays a crucial role in steroid drug production. Hydroxylation, an essential physiological mechanism in mammals, is involved in the activation of pro-drugs and detoxification of exogenous steroids. Androstenedione can be synthesized from dehydroepiandrosterone (DHEA) and further converted into testosterone or estrone through specific enzymatic reactions. Various tissues within the body, such as the adrenal cortex and liver, contribute to the synthesis and metabolism of androstenedione. The metabolism of androgens, including androstenedione, is regulated by hormone activities in vivo, presenting challenges for studying their metabolism within bone. Peripheral tissues extensively metabolize circulating androgens, which are then excreted in urine. Enzymes such as hydroxysteroid dehydrogenases, reductases, and conjugation enzymes play significant roles in the metabolism of androgens. Furthermore, androstenedione can be converted into various metabolites, including testosterone, estradiol, and estrone, depending on tissue-specific enzyme activity. In some cases, androstenedione can be directly converted into potent estrogens or androgens without forming testosterone. Understanding the metabolism of androstenedione is crucial as gene polymorphisms encoding key enzymes involved in these pathways can modulate endogenous hormone levels and potentially influence the risk of hormone-related diseases. 2
Reference
1. Malaviya A, Gomes J. Androstenedione production by biotransformation of phytosterols. Bioresour. Technol. 2008;99:6725–6737.
2. Badawy MT, Sobeh M, Xiao J, Farag MA. Androstenedione (a Natural Steroid and a Drug Supplement): A Comprehensive Review of Its Consumption, Metabolism, Health Effects, and Toxicity with Sex Differences. Molecules. 2021;26(20):6210.
Related articles And Qustion
See also
Lastest Price from Androstenedione manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 63-05-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000KG

US $40.00/kg2025-03-07
- CAS:
- 63-05-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons