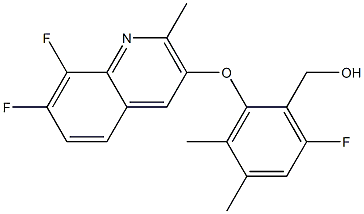
What kind of fungicide is Ipflufenoquin?
Description
Ipflufenoquin was announced by Nippon Soda as a new fungicide in 2017. Based on structural similarities to quinofumelin and also on the fact that both active ingredients share the same target spectrum (control of Botryotinia fuckeliana and other mold diseases of fruits and vegetables, but also Pyricularia oryzae, the causal agent of rice blast), it can be assumed that both active ingredients share the same mode of action.
Olorofim and ipflufenoquin
The Fungicide Resistance Action Committee (FRAC) has classified ipflufenoquin as a DHODH inhibitor, meaning ipflufenoquin shares its MoA with olorofim. Furthermore, the risk of resistance selection by ipflufenoquin is moderate to high, suggesting a potential human health concern. Preliminary in vitro susceptibility testing of 21 Aspergillus isolates indicated that olorofim and ipflufenoquin display in vitro activity against all but four and five Aspergillus spp. isolates, respectively. Based on drug concentration (mg/L), olorofim was more potent against Aspergillus (MIC50 0.016/0.06 mg/L with 50 % and complete inhibition endpoints, respectively) than ipflufenoquin (MIC50 2/4 mg/L)[1]. These data suggest an overall similarity between the medical DHODH inhibitor olorofim and the agricultural pesticide ipflufenoquin against Aspergillus spp., supporting the concern that ipflufenoquin use may cause cross-resistance to olorofim in Aspergillus.
Synthesis method
![Benzenemethanol,2-[(7,8-difluoro-2-methyl-3-quinolinyl)oxy]-6-fluoro-,-dimethyl- Article illustration](/NewsImg/2024-02-19/6384393089492989632176625.png)
The synthesis of ipflufenoquin starts with the transformation of 2,3-difluoroaniline (86) with chloral hydrate and hydroxylamine hydrochloride, which delivers via the formation of the reactive α -oximinoamide intermediate 87 the difluorinated isatin derivative 88. Ring enlargement of this indoline-2,3-dione with bromoacetone leads to the tetrasubstituted quinoline carboxylic acid 89. Its thermal decarboxylation affords the quinolinol 90, which, upon treatment with 2,6-difluoro acetophenone in the presence of a base, yields the phenyl quinolinyl ether 91. The latter is converted into ipflufenoquin by Grignard-type addition of methylmagnesium chloride to the ketone function[2].
References
[1] Paul E. Verweij . “Dual use of antifungals in medicine and agriculture: How do we help prevent resistance developing in human pathogens?” Drug Resistance Updates 65 (2022): Article 100885.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.