Analysis of the application effect of vinpocetine in neurological diseases
Introdution
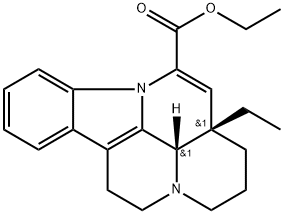
Vinpocetine (chemical name 14-ethoxycarbonyl-(3a,16a-ethyl)-14,15-eburnamine) is a synthetic derivative of vinca alkaloid vincamine, which is an alkaloid extracted from the periwinkle plant, Vinca minor (Figure. 1). Vinpocetine can pass the blood-brain barrier and enter the brain after oral or intravenous administration. The chemical structure, pharmacokinetics, metabolism, and distribution have been previously reviewed in detail.[1] Vinpocetine, trade name as Cavinton, was originally developed and marketed in Hungary around 1978. Vinpocetine has been initially developed for the treatment of neurological diseases associated with cerebrovascular disorders such as stroke and dementia that are often caused by ischemia or other cognitive deficits. A number of studies have reported the protective effects of vinpocetine after ischemic injury of the brain in rodents.[2]

Clinical applications in neurological diseases
Vinpocetine has successfully been used in the treatment of central nervous system disorders of cerebrovascular origin for decades. The increase in the regional cerebral blood flow in response to vinpocetine administration is well established and strengthened by new diagnostical techniques (transcranial Doppler, near infrared spectroscopy, positron emission tomography). The latest in vitro studies have revealed the effect of the compound on Ca2+/calmodulin dependent cyclic guanosine monophosphate-phosphodiesterase 1, voltage–operated Ca2+ channels, glutamate receptors and voltage dependent Na+-channels; the latest being especially relevant to the neuroprotective action of vinpocetine. The good brain penetration profile and heterogenous brain distribution pattern (mainly in the thalamus, basal ganglia and visual cortex) of labelled vinpocetin were demonstrated by positron emission tomography in primates and man. Multicentric, randomized, placebocontrolled clinical studies proved the efficacy of orally administered vinpocetin in patients with organic psychosyndrome. Recently positron emission tomography studies have proved that vinpocetine is able to redistribute regional cerebral blood flow and enhance glucose supply of brain tissue in ischemic post-stroke patients.[1] The following is an analysis of the efficacy of the combination use of vinpocetine and other drugs.
1. Analysis of the Effect of Aspirin Combined with Vinpocetine in the Treatment of Cerebral Infarction
To explore the application value of aspirin combined with vinpocetine in the treatment of cere‐ bral infarction. A total of 92 patients with cerebral infarction admitted to Shunde Hospital of Southern Medical University Affiliated Xingtan Hospital from January 2021 to December 2023 were simply and conveniently selected as the research objects, and they were divided into two groups according to different treatment schemes, with 46 cases in each group. The control group was treated with aspirin, and the observation group was treated with aspirin combined with vinpocetine. The effective rate, neurological deficit score and daily activity ability of the two groups were compared. After treatment, the National Institute of Health Stroke Scale score and Modified Rankin Scale score in the observation group were lower than those in the control group, and the activity of daily living score was higher than that in the control group, the differences were statistically significant (all P<0.05). The total effective rate of the observation group was 93.48% (43/46), which was higher than 78.26% (36/46) of the control group, and the difference was statistically significant (χ2 =4.389, P=0.036). The combination of aspirin combined with vin‐ pocetine can promote the early recovery of neurological function in patients with cerebral infarction, and can improve the total effective rate.[3]
2.Effects of vinpocetine combined with clopidogrel on patients with acute cerebral infarction
To observe effects of vinpocetine combined with clopidogrel on patients with acute cerebral infarction. A prospective study was conducted on 100 patients with acute cerebral infarction admitted to this hospital from January 2020 to December 2020. They were divided into control group and study group according to the random number table method, 50 cases in each. The control group was treated with clopidogrel, while the study group was treated with vinpocetine on the basis of that of the control group. The clinical efficacy, the vascular endothelial function index levels [endothelin (ET)-1, nitric oxide (NO)], the inflammatory factor levels [C-reactive protein (CRP), interleukin (IL)-6, IL-8, IL-10] levels, the neurological deficit degree [National Institutes of Health stroke scale (NIHSS)] score, the activities of daily living [activity of daily living scale (ADL)] score, and the incidence of adverse reactions were compared between the two groups. The total effective rate of treatment in the study group was 98.00% (49/50), which was higher than 78.00% (39/50) in the control group, and the difference was statistically significant (P<0.05). After the treatment, the levels of ET-1 in the two groups were lower than those before the treatment, and that in the study group was lower than that in the control group; the NO levels of the two groups were higher than those before the treatment, and that in the study group was higher than that in the control group; and the difference was statistically significant (P<0.05). The levels of CRP, IL-6, IL-8 and IL-10 in the two groups were lower than those before the treatment, those in the study group were lower than those in the control group, and the differences were statistically significant (P<0.05). After 7 and 14 days of treatment, the NIHSS scores of the two groups were lower than those before the treatment, those in the study group were lower than those in the control group, and the differences were statistically significant (P<0.05). The ADL scores of the two groups were higher than those before the treatment, those in the study group were higher than those in the control group, and the differences were statistically significant (P<0.05). During the treatment, there were no obvious adverse reactions in the two groups. Vinpocetine combined with clopidogrel in the treatment of the patients with acute cerebral infarction can improve the total effective rate and the ADL scores, improve the levels of vascular endothelial function indexes, reduce the levels of inflammatory factors and the NIHSS scores. Moreover, it is superior to single clopidogrel treatment.[4]
Conclusion
In the brain, vinpocetine improves brain blood flow by acting as a cerebral vasodilator ; and enhances cerebral metabolism by increasing oxygen and glucose uptake and stimulating neuronal ATP production. In a number of neuronal cells or nerve terminals, vinpocetine has also been shown to function as an antioxidant and prevent neurotoxic calcium and sodium elevation. Thus, vinpocetine has become one of the most commonly used adjuvant drugs for neurological diseases. No significant side effects and toxicity have been reported for vinpocetine at therapeutic doses and it is generally thought to be safe for long-term use.[2]
References
[1] Bönöczk P, Gulyás B, Adam-Vizi V, et al. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Res Bull. 2000;53(3):245-254.
[2] Zhang YS, Li JD, Yan C. An update on vinpocetine: New discoveries and clinical implications. Eur J Pharmacol. 2018;819:30-34.
[3] Zeng Sw, Huang Zm, Ni Wk, etal. Analysis of the Effect of Aspirin Combined with Vinpocetine in the Treatment of Cerebral Infarction [J]. China &Foreign Medical Treatment, 2024;43(28):84-86+106.
[4] Wang Yx. Effects of Vinpocetine combined with Clopidogrel on patients with acute cerebral infarction [J].Medical Journal of Chinese People's Health,2023;35(02):12-14+18.
You may like
Lastest Price from Vinpocetine manufacturers

US $5.00-0.50/KG2025-05-09
- CAS:
- 42971-09-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/kg2025-04-27
- CAS:
- 42971-09-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg