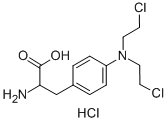
An Introduction to Melphalan and Its Mechanism of Carcinogenesis
General Description
Melphalan, sold under the brand name Alkeran among others, is a chemotherapy medication used to treat multiple myeloma, ovarian cancer, melanoma, and AL amyloidosis. Melphalan is an antineoplastic agent that acts as a bifunctional alkylating agent. It is used in the treatment of multiple myeloma, advanced ovarian adenocarcinoma, early and advanced breast cancer, childhood neuroblastoma, and polycythaemia vera. Melphalan is also used for regional arterial perfusion in localized malignant melanoma, and localized soft-tissue sarcoma of the extremities.1
Figure 1. Properties of Melphalan
Mechanism of Action
Melphalan can act either as monofunctional (one alkylating moiety) or bifunctional (two alkylating moieties). Bifunctional melphalan has the capacity to crosslink both interstrand and intrastrand in DNA. After administration, alkylating agents get converted into carbonium ions, and results in alkylation at N7 and O6 of guanine in malignant cells DNA. Three different mechanisms of alkylating agents can be elucidated as follows:
1.Melphalan damages the DNA by alkylation and crosslinking between DNA strands, which eventually inhibits DNA transcription and leads to cell death.
2.Mutations in DNA strand is another approach that results in the abnormal pairing of nucleotides.
3.Melphalan confers its alkyl group to bases of DNA. Thereafter, DNA repair enzymes try to swap the attached alkyl group, which leads to fragmentation of DNA and cell death.
Melphalan can act in both the resting and cycling stage. However, proliferating cells are more attracted to drugs in G1 and S phases.2-3
Mechanism of Carcinogenesis
Induction of DNA damage
Melphalan is a direct-acting, bifunctional alkylating agent that binds to cellular macromolecules. As a phenylalanine derivative of nitrogen mustard, it is capable of producing a variety of DNA adducts including mono-adducts at the N7 of guanine and the N3 of adenine as well as interstrand cross-links, and pre-mutagenic lesions that are believed to play a critical role in its toxic and carcinogenic effects. Melphalan has been shown to bind to DNA, RNA, and protein in cells in vitro, and DNA-binding has been seen in rats treated in vivo. The formation of DNA cross-linking in vitro and in vivo has also been reported based on the measurement of specific adducts or by changes in DNA migration in DNA strand breakage or electrophoretic assays.4
Mutational consequences of DNA damage
Melphalan has been tested for genotoxicity in an assortment of short-term assays, both in vitro and in vivo. Increased frequencies of dominant lethal mutations, chromosomal aberrations, micronuclei, and DNA strand breaks have been observed in several studies following treatment of rodents with melphalan. In the mouse-specific locus mutation and heritable translocation tests, increases in mutations were seen in both spermatagonial as well as postspermatogonial germ cells. The observed mutations originated primarily from large deletions in the postspermatogonial cells whereas other types of mutagenic mechanisms predominated in the spermatogonial cells.5
Melphalan also induced chromosomal aberrations, sister chromatid exchange, micronuclei, mutations at the HPRT gene, and DNA damage in human cells in vitro. It also induced transformation of C3H 10T1/2 and other cells. In cultured rodent cells, it induced chromosomal aberrations, sister chromatid exchange, gene mutations, and DNA damage. In addition, it induced aneuploidy and sex-linked recessive lethal mutations in Drosophila, and mutation in bacteria.6
Absorption, Distribution, Metabolism, and Excretion
In humans, following oral administration, melphalan is absorbed from the gastrointestinal tract with a wide range in bioavailability (range 25–89%, mean 56%). It also exhibits considerable variability with respect to the time of its appearance in the plasma (range ~0–6 hours), and in the peak plasma concentration achieved (range 70–4000 ng/mL, depending upon the dose). This variability may be due to incomplete intestinal absorption, variable first-pass metabolism, and/or rapid hydrolysis. Upon absorption, approximately 60–90% of plasma melphalan is bound to plasma proteins such as albumin, and to a lesser degree, α1-acid glycoprotein, with approximately 30% being bound irreversibly. Melphalan does not undergo metabolic activation and is inactivated in the plasma, primarily by non-enzymatic hydrolysis to monohydroxymelphalan and dihydroxymelphalan. Apart from these hydrolysis products, no other melphalan metabolites have been detected in humans. Melphalan enters cells through active transport, mostly by the high-affinity l-amino acid transport system, which carries glutamine and leucine.7
As a consequence of its inconsistent absorption, melphalan can also exhibit considerable variability in its elimination. For example, when given intravenously, melphalan exhibited a fairly consistent half-life (14–40 minutes in a study of ten patients given a 20 mg/m2 dose) but was absorbed variably, and found to have a half-life of 36–552 minutes in a study of 13 patients administered a 0.6 mg/m2 oral dose. About 10% of the drug is excreted unchanged in the urine within 24 hours, and about 30% of administered melphalan (including metabolites) is excreted in the urine within 9 days of oral administration. Approximately 20–50% of the dose is eliminated via faeces.8
Synthesis
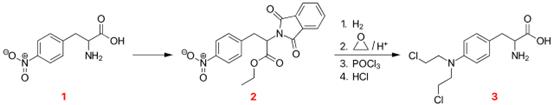
Figure 2. Properties of Melphalan
4-Nitro-L-phenylalanine 1 was converted to its phthalimide by heating with phthalic anhydride, and this was converted to its ethyl ester 2. Catalytic hydrogenation produced the corresponding aniline. Heating in acid with oxirane, followed by treatment with phosphorus oxychloride provided the bischloride, and removal of the protecting groups by heating in hydrochloric acid gave melphalan 3.9
References
1.R.L. Furner, R.K. Brown, L-Phenylalanine mustard (L-PAM): the first 25 Years, Cancer Treat Rep. 64 (1980) 559–574.
2.R. Ralhan, J. Kaur, Alkylating agents and cancer therapy, Expert Opin. Ther. Pat.17 (2007) 1061–1075.
3.K.W. Kim, J.K. Roh, H.J. Wee, C. Kim, in: Alkylating Anticancer Drugs, Cancer Drug Discovery, 2016, pp. 71–94.
4.GlaxoSmithKline (2007).Prescribing information leaflet: Alkeran (melphalan) tablets.
5.Witt KL, Bishop JB. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat Res. 1996;355:209–234.
6.Allan JM, Engelward BP, Dreslin AJ, et al. Mammalian 3-methyladenine DNA glycosylase protects against the toxicity and clastogenicity of certain chemotherapeutic DNA cross-linking agents. Cancer Res. 1998;58:3965–3973.
7.Nieto Y, Vaughan WP. Pharmacokinetics of high-dose chemotherapy. Bone Marrow Transplant. 2004;33:259–269.
8.McEvoy GK, editor (2007). American Hospital Formulary Service Drug Information. Bethesda, MD: American Society of Health-System Pharmacists.
9.Bergel F, Stock JA (1954). "Cyto-active amino-acid and peptide derivatives. Part I. Substituted phenylalanines". Journal of the Chemical Society (Resumed): 2409.
Lastest Price from 4-BIS(2-CHLORETHYL)-AMINO-L-PHENYLALANINE HYDROCHLORIDE manufacturers

US $5.00-0.50/KG2025-05-29
- CAS:
- 3223-07-2
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 3223-07-2
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 100kg