The introduction of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II)
General description
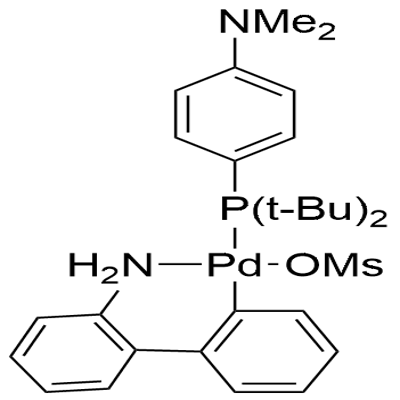
The Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II), with the CAS No: 1820817-64-8, is also known as Amphos Palladacycle Gen. 3. This chemical’s molecular formula is C29H41N2O3PPdS and molecular weight is 635.11. Its melting point is 192-201 °C (decomposition) and it is a beige to tan powder. It is a kind of mental catalyst. Its structure is as follows:

Figure 1 Appearance of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II).
Chemical synthesis of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II)
Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) can be synthesized by single step according to the previous work [1]. Amphos Pd G3 was synthesized with reference to the synthesis of other G3 complexes such as SPhos Pd G3 and XPhos Pd G3. [2] μ-OMs dimer (763 mg, 1.03 mmol), which was prepared by the reported procedure [2], and 4-(di-tert-butylphos- phino)-N,N-dimethylaniline (Amphos; 548 mg, 2.06 mmol) were placed in a 25 mL Schlenk tube. After evacuation and backfilling with argon, THF (15 mL) was added. The resulting dark brown solution was stirred at room temperature for 30 min. About 90% of the solvent was concentrated in vacuo, and then, the residue was triturated with pentane to give Amphos Pd G3 as a pale-yellow solid. Yield 1.05 g (1.65 mmol, 80%). 1H NMR (500 MHz, CDCl3): δ 1.13 (d, J = 14.0 Hz, 9H), 1.29 (d, J = 13.8 Hz, 9H), 2.74 (s, 3H), 2.97 (s, 6H), 4.72 (br, 1H), 6.53 (d, J = 7.7 Hz, 2H), 6.60 (td, J = 7.6, 0.9 Hz, 1H), 6.81 (dd, J = 8.0, 4.0 Hz, 1H), 6.92 (t, J = 7.3 Hz, 1H), 7.17?7.21 (m, 2H), 7.27 (td, J = 7.5, 1.3 Hz, 1H), 7.41 (br t, J = 8.6 Hz, 2H), 7.45? 7.49 (m, 2H), 7.53 (br d, J = 6.3 Hz, 1H); 31P{1H} NMR (200 MHz, CDCl3): δ 64.
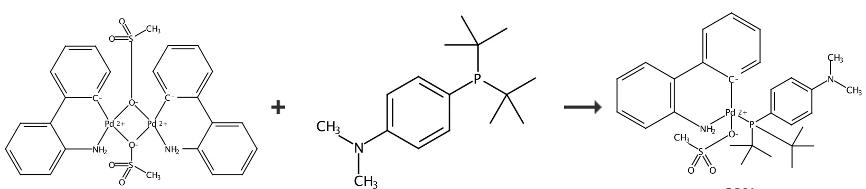
Figure 2 Chemical synthesis of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II).
Application of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II)
Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) is a kind of Palladium catalyst. Palladium-mediated cross-coupling reactions to form C?C, C?N, C?O, and C?S bonds (e.g., Buchwald?Hartwig aminations) are among the most powerful organometallic transformations employed in organic synthesis [3]. Over the past thirty years palladium-catalyzed coupling reactions have become powerful tools for generating carbon–carbon and carbon–heteroatom bonds [2]. The catalytic reaction cycle typically involves three steps: (i) oxidative addition of Pd0 with aryl halide, (ii) formation of palladium(II) complex through transmetalation, and (iii) regeneration of the active Pd0 catalyst through reductive elimination. Pd(II) sources, such as Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) must be reduced in situ.
Mass spectrometric pattern of Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II)
The Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) intermediates can be detected by desorption electrospray ionization according to the previous work [3]. Upon mixing Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) in THF, a major ion corresponding to the cation of it at m/z 539 appeared in the acquired desorption electrospray ionization spectrum (DESI-MS). Figure 3 shows that once NaOtBu in MeOH was introduced and online mixed with Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II), the sodium-adducted Pd0L species at m/z 394 was formed and immediately captured by MS. It is reasonable that sodiated Pd0L rather than the protonated Pd0L was detected because the reaction mixture has excess NaOtBu. The inset in Figure 3 clearly shows the zoomed-in spectrum of [Pd0L + Na]+ at m/z 394, which exactly matches its theoretical isotopic peak distribution. With the ability to detect the catalytically reactive Pd0L species using the DESI-MS approach, the ability to detect other species is investigated within the reaction cycle. Premixing Methanesulfonato{[4-(N,N-Dimethylamino)Phenyl]Di-T-Butylphosphino}(2'-Amino-1,1'-Biphenyl-2-Yl)Palladium(II) with iodobenzene (PhI), and further mixing with NaOtBu, allowed the in situ generated Pd0L species to undergo oxidative addition with PhI to produce [LPdPh]+, which was successfully observed at m/z 448. Besides the observation of Pd0L (m/z 394) and [LPdPh]+ (m/z 448), generation of a new set of peaks at m/z 647 indicated the successful formation and detection of the PdII-amine complex, [LPdIIPh(HN2R)]+.
References
[1]Masuda et al. Late-Stage Functionalization of the Periphery of Oligophenylene Dendrimers with Various Arene Units via Fourfold C-H Borylation. Journal of Organic Chemistry, 2021, 86(21): 14433-14443.
[2]Bruno et al. Design and preparation of new palladium precatalysts for C-C and C-N cross- coupling reactions. Chem. Sci. 2013, 4, 916?920.
[3]Zheng et al. Capture of Reactive Monophosphine-Ligated Palladium(0) Intermediates by Mass Spectrometry. Journal of the American Chemical Society, 2015, 137(44): 14035-14038.
Related articles And Qustion
See also
Lastest Price from APhos Pd G3 manufacturers
![1820817-64-8 Methanesulfonato{[4-(N,N-dimethylamino)phenyl]di-t-butylphosphino}(2'-amino-1,1'-biphenyl-2-yl)palladium(II), min. 98 [Amphos Palladacycle Gen. 3]](/ProductImageEN1/2025-06/Small/fbbd69a2-3a74-4874-b95a-35b1d5ae4146.png)
US $2.00-5.00/kg2025-06-20
- CAS:
- 1820817-64-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg