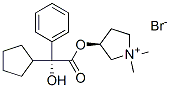
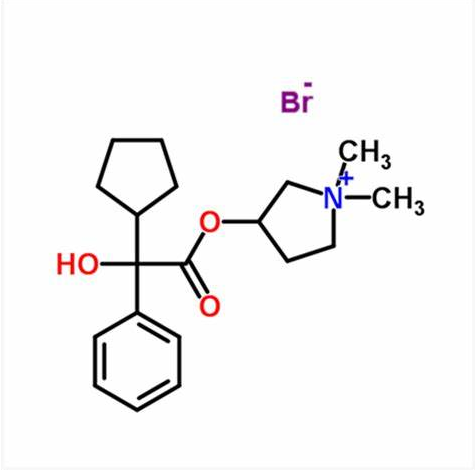
Glycopyrronium: Interactions, Mechanism of Action and ADME Profiles
General Description
Glycopyrronium bromide, also known as NVA237 or glycopyrrolate, is a racemic mixture of two enantiomers.They are both quaternary ammonium compounds and long acting muscarinic antagonists. It is one of the most commonly prescribed anticholinergic medications. Early research into glycopyrronium use was for its indication as an adjunct therapy in the treatment of peptic ulcers. Later research, taking advantage of the systemic distribution of muscarinic receptors through the body, found that glycopyrronium could also be used for reducing sweat gland, oral, airway, and gastric secretions; as well as reducing cardiac inhibitory reflexes; and reducing bronchoconstriction in COPD. Glycopyrronium is commonly prescribed as a first line treatment for a wide variety indications and is considered to have a wider therapeutic window than tiotropium.Glycopyrronium was originally granted FDA approval on 11 August 1961.1-3
Figure 1. Properties of Glycopyrronium Bromide Tablets
Indication
Glycopyrronium formulated as a topical cloth is indicated to treat primary axillary hyperhidrosis in patients ≥9 years, and an inhalational solution is indicated for long term maintenance of airflow obstruction in COPD. A glycopyrronium intravenous and intramuscular injection is indicated in adults and pediatric patients to reduce the volume and acidity of gastric secretions, reduce airway secretions, and block cardiac inhibitory reflexes during the induction of anesthesia and intubation; to treat surgically-induced, drug-induced, or vagal reflex associated arrhythmias intraoperatively; and to prevent peripheral muscarinic effects of cholinergic drugs. The same injection is indicated in adults as an adjunct therapy in the treatment of peptic ulcers, as is an orally disintegrating tablet formulation. An oral solution is indicated to treat excessive drooling associated with neurologic conditions in patients aged 3-16 years. Glycopyrronium and budesonide19 can be formulated with formoterol fumarate for the maintenance of COPD.4-5
Mechanism of Action
Glycopyrronium is a muscarinic antagonist with the highest affinity for M1 receptors, followed by M3, M2/M4, and M5. Muscarinic receptors M1 to M4 are found in the lung, although M3 is predominantly responsible for bronchoconstriction and airway secretions. Secretions from salivary and sweat glands, as well as gastric acid secretions, are also predominantly mediated by the M3 receptor. Salivary and gastric acid secretions are also partially mediated by the M1 receptor. Antagonism of these receptors decreases the volume of their respective secretions, and in the case of the gastrointestinal system, reduces the acidity of the stomach. In the cardiovascular system, muscarinic receptors M1 to M5 are all present, however the function of M5 has not been described in literature. Under normal circumstances, stimulation of the vagal nerve lowers the heart rate, potentially leading to intraoperative bradycardia. Studies in mice suggest that this stimulation is predominantly mediated by the M3 receptor, and mutant knockout mice are not susceptible to these effects.6
ADME Profiles
Pharmacokinetics
Glycopyrronium affects the gastrointestinal tracts, liver and kidney but has a very limited effect on the brain and the central nervous system. In horse studies, after a single intravenous infusion, the observed tendencies of glycopyrronium followed a tri-exponential equation, by rapid disappearance from the blood followed by a prolonged terminal phase. Excretion was mainly in urine and in the form of an unchanged drug. Glycopyrronium has a relatively slow diffusion rate, and in a standard comparison to atropine, is more resistant to penetration through the blood-brain barrier and placenta.7
Pharmacodynamics
Glycopyrronium is a quaternary ammonium compound that is one of the most commonly prescribed long acting muscarinic antagonists. Glycopyrronium slowly dissociated from muscarinic receptors, leading to a long duration of action. It has a wider therapeutic index than other anticholinergic medications, such as tiotropium. Patients should be counselled regarding the risk of worsening urinary retention, risk of overheating, and transient blurred vision.8
Metabolism
Glycopyrronium is hydrolyzed to the inactive M9 metabolite. Metabolism was mainly mediated by CYP2D6, with minor contributions from CYP1A2, CYP2B6, CYP2C9, CYP2C18, CYP2C19, and CYP3A4.9
Absorption
In adults, a 66 mg topical dose of glycopyrronium reaches a Cmax of 0.08 ± 0.04 ng/mL, with a Tmax of 1 hour, and an AUC0-24 of 0.88 ± 0.57 h*ng/mL. Inhaled glycopyrronium is approximately 40% bioavailable. A 25 µg inhaled solution reaches a Cmax of 34.5 pg/mL, with a Tmax of <20 minutes, and an AUC0-inf of 255 h*pg/mL. An 8 µg/kg intramuscular dose reaches a Cmax of 3.47 ± 1.48 µg/L, with a Tmax of 27.48 ± 6.12 minutes, and an AUC of 6.64 ± 2.33 h*g/L. Oral glycopyrronium has highly variable pharmacokinetics, reaching a mean Cmax of 0.318 ng/mL, a Tmax of 3.1 hours, and an AUC0-24 of 1.74 h*ng/mL.10
Half-life and Clearance
The half life after inhalation is approximately 33-53 hours. The mean half life of a 6 µg/kg intravenous dose is 0.83 ± 0.27 hours. The mean half life of oral glycopyrronium is 3.0 hours. A 6 µg/kg intravenous dose has a clearance of 0.54 ± 0.14 L/kg/h. An oral solution has a clearance of 5.28-38.95 L/h/kg in healthy adults and 8.07-25.65 L/h/kg in patients with cerebral palsy.10
Volume of distribution
The mean volume of distribution in patients aged 1-14 years old is 1.3-1.8 L/kg, with a range of 0.7-3.9 L/kg. The volume of distribution in adults aged 60-75 years is 0.42 ± 0.22 L/kg.11
Protein binding
Glycopyrronium is 38-44% protein bound in plasma. It is bound to serum albumin, alpha-1-acid glycoprotein, as well as other plasma proteins that have not been identified in literature.9
Toxicity
Patients presenting with an overdose typically present with flushing, hyperthermia, tachycardia, ileus, urinary retention, loss of ocular accommodation, light sensitivity, mydriasis, nausea, vomiting, dizziness, light headedness, and obstipation.Patients should be treated with symptomatic and supportive therapy, which may include the use of catheters for urinary retention, cardiovascular support, airway maintenance, ventilation, or neostigmine. The oral LD50 in mice is 570 mg/kg, and in rats is 709 mg/kg.14 The intraperitoneal LD50 in mice is 90 mg/kg, and in rats is 196 mg/kg.11
Synthesis
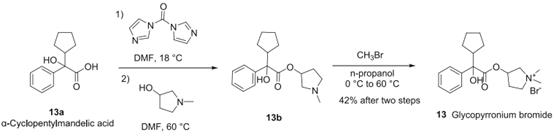
Figure 2. Synthesis of Glycopyrronium bromide
The synthetic process for glycopyrronium bromide starts with the esterification of cyclopentyl mandelic acid. To the acid 13a dissolved in DMF solvent, carbonyldiimidazole was added at 18 oC. Subsequently, 1-methyl-pyrrolidin-3-ol was added, and the reaction mixture was heated to 60 oC. After completion of the reaction, water was added to the reaction mixture and extracted with toluene to obtain ester 13b as a 50% solution in toluene. In the next step, 50% 13b toluene solution was diluted with n-propanol followed by methyl bromide addition at 0 oC with gradual heating to evaporate excess alkyl halide. Upon slow cooling to 15 oC, the solid starts to crystallize out. The major portion of this crystallized solid consists of 3S,2R, and 3R,2S diastereomeric mixture up to 90%, whereas 3S,2S, and 3R,2R diastereomeric pair remain soluble in n-propanol. Further recrystallization of solid in n-propanol enriches the 3S,2R, and 3R,2S diastereomeric excess to >99.9%.12
References
1.FDA Assessment Report: Seebri Neohaler (Glycopyrronium) Inhalation Powder
2.Reid SM, Westbury C, Guzys AT, Reddihough DS: Anticholinergic medications for reducing drooling in children with developmental disability. Dev Med Child Neurol. 2020 Mar;62(3):346-353.
3.Kirchertz R, Hilbert T: A week of slow hearts: anaesthesia for eye surgery and shortage of glycopyrrolate. Minerva Anestesiol. 2019 Sep;85(9):1033-1034.
4.FDA Approved Drug Products: Glyrx-PF (Glycopyrronium) Intravenous or Intramuscular Injection.
5.FDA Approved Drug Products: Bevespi Aerosphere (Glycopyrronium and Formoterol Fumarate) Inhalation Aerosol.
6.Saternos HC, Almarghalani DA, Gibson HM, Meqdad MA, Antypas RB, Lingireddy A, AbouAlaiwi WA: Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 2018 Jan 1;50(1):1-9.
7.Rumpler, M.J.; Colahan, P.; Sams, R.A. (2014). "The pharmacokinetics of glycopyrrolate in Standardbred horses". Journal of Veterinary Pharmacology and Therapeutics. 37(3): 260–8.
8.Sykes DA, Dowling MR, Leighton-Davies J, Kent TC, Fawcett L, Renard E, Trifilieff A, Charlton SJ: The Influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012 Nov; 343(2): 520-8.
9.EMA Assessment Report: Seebri Breezhaler (Glycopyrronium) Inhalation Powder.
10.FDA Approved Drug Products: Cuvposa (Glycopyrronium) Oral Solution.
11.FDA Approved Drug Products: Qbrexza (Glycopyrronium Tosylate) Topical Cloth.
12.Kazi, A. A., Reddy, B. S., & Singh, L. R. (2021). Synthetic approaches to FDA approved drugs for asthma and COPD from 1969 to 2020. Bioorganic & Medicinal Chemistry,41, 116212.
Related articles And Qustion
See also
Lastest Price from Glycopyrronium Bromide manufacturers

US $0.00-0.00/G2025-04-21
- CAS:
- 51186-83-5
- Min. Order:
- 100G
- Purity:
- 99%
- Supply Ability:
- 20TONS

US $115.00/g2025-03-26
- CAS:
- 51186-83-5
- Min. Order:
- 100g
- Purity:
- 98%
- Supply Ability:
- 100kg